Benzyl iodide
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
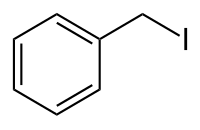
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Benzyl iodide | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 7 H 7 I. | |||||||||||||||
| Brief description |
colorless to yellow needles |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 218.035 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.7335 g cm −3 (25 ° C) |
|||||||||||||||
| Melting point |
24.5 ° C |
|||||||||||||||
| boiling point |
93 ° C (10 Torr ) |
|||||||||||||||
| solubility |
very soluble in benzene, ether and ethanol |
|||||||||||||||
| Refractive index |
1.6334 (25 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Benzyl iodide is an organic-chemical compound from the group of alkyl halides and has the empirical formula C 7 H 7 I. It is a constitutional isomer to the iodotoluenes .
Manufacturing
Benzyl iodide can be produced from benzyl chloride and sodium iodide in acetone by the Finkelstein reaction .
properties
Benzyl iodide forms colorless to yellow needles that melt at 24.5 ° C. As a liquid, it has a high refractive index of 1.6334. Benzyl iodide is a strong eye irritant, already 0.002 mg per liter of air causes tearing.
Individual evidence
- ↑ a b c d e f g h David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-306.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ Fieser and Fieser: Organic Chemistry , 2nd Edition, Verlag Chemie, Weinheim 1982, ISBN 3-527-25075-1 .
