Bevirimat
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
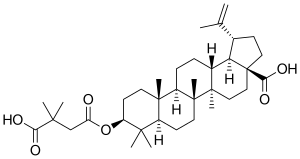
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Bevirimat | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 36 H 56 O 6 | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 584.83 g · mol -1 | |||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Bevirimat (PA-457) is an experimental drug that is being developed to treat HIV- infected patients as part of combination therapy for HIV .
It belongs to the group of maturation inhibitors .
history
After the first results of a small study on HIV patients were published in 2005, the data of a placebo-controlled IIa study were published at the end of 2005. The patients received oral monotherapy with PA-457 for ten days. In the highest-dose 200 mg arm, an average decrease in viral load of 1.03 log levels was achieved. In the 100 mg group it was still 0.48 log levels. There were also patients in whom no significant effects on viral load could be demonstrated.
pharmacology
Bevirimat is a derivative of betulinic acid , which can be isolated as triterpene carboxylic acid from birch bark. Bevirimat inhibits replication in a late phase of the reproduction cycle, the so-called budding or maturation of the virions . Bevirimat interrupts the conversion of the capsid precursor (p25) into the mature capsid protein (p24). Non-infectious viruses develop. Bevirimat works synergistically with other antiviral substances.
Bevirimat was well tolerated in studies.
Pharmacokinetics
The substance has a long half-life, so that a single daily dose is sufficient.
Resistances
Bevirimat is also effective against resistant viruses. So far, resistance has not occurred in humans. Resistance mutations in the capsid and Gag target genes could be selected in the laboratory. These were point mutations. Therefore, a low resistance barrier is feared. However, resistant mutants seem to have a lower replication fitness than wild-type viruses. In January 2010 a study was published to predict Bevirimat resistance based on the genotype of HIV-1.
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Martin D, et al. The safety, tolerability, and pharmacokinetics of multiple oral doses of PA-457, the first-in-class HIV maturation inhibitor, in healthy volunteers. Abstract 551th 12th CROI 2005, Boston.
- ↑ Beatty G, Jacobson J, Lalezari J, et al. Safety and Antiviral Activity of PA-457, the First-In-Class Maturation Inhibitor, in a 10-Day Monotherapy Study in HIV-1 Infected Patients. Abstract H-416D, 45th ICAAC 2005, Washington.
- ↑ Li F, R Goila-Gaur, Salzwedel K, et al. PA-457: a potent HIV inhibitor that disrupts core condensation by targeting a late step in gag processing. Proc Natl Acad Sci USA 2003, 100: 13555-60. PMID 14573704 .
- ↑ Kilgore N, Reddick M, Zuiderhof M, et al. The first-in-class maturation inhibitor, PA-457, is a potent inhibitor of HIV-1 drug-resistant isolates and acts synergistically with approved HIV drugs in vitro. Abstract 509, 13th CROI 2006, Denver.
- ↑ a b Adamson C, Salzwedel K, Castillo A, et al. Viral resistance to PA-457, a novel inhibitor of HIV-1 maturation. Abs. 156, 13th CROI 2006, Denver.
- ↑ Smith P, Forrest A, Beatty G, et al. Pharmacokinetics / pharmacodynamics of PA-457 in a 10-day multiple dose monotherapy trial in HIV-infected patients. Abstract 52, 13th CROI 2006, Denver.
- ↑ Dominik Heider , Jens Verheyen, Daniel Hoffmann: Predicting Bevirimat resistance of HIV-1 from genotype . In: BMC Bioinformatics . 11, No. 1, January 20, 2010, p. 37. doi : 10.1186 / 1471-2105-11-37 . Retrieved June 30, 2012.