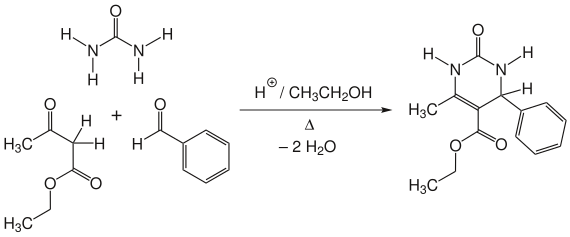Biginelli reaction
The Biginelli reaction is a reaction from the field of organic chemistry and is a multi-component reaction . The Italian chemist Pietro Biginelli (1860–1937) from the University of Florence first reported on the Biginelli reaction in 1893. It is an acid-catalyzed cyclocondensation of acetoacetic ester , benzaldehyde and urea . This produces a 3,4-dihydropyrimidin-2 (1 H ) -one:
application
The scope of this heterocycle synthesis is significantly greater. All three starting materials can be varied widely, so that a large number of different multiply functionalized pyrimidine derivatives are accessible via the Biginelli reaction. Instead of benzaldehyde , aromatic aldehydes substituted in the ortho , meta or para position can also be used. The reaction also proceeds smoothly with heteroaromatic aldehydes. When using aliphatic aldehydes, the reaction yields are usually lower. Derivatives of aldoses can also be used as the aldehyde component. Instead of acetoacetic esters, many other CH-acidic compounds, such as 3-oxocarboxylic acid thioesters, 1,3-diketones, etc., can be used as starting materials in the Biginelli reaction. The urea component can also be varied. The Biginelli reaction also works with thiourea, substituted ureas and thioureas, and with guanidines.
The Biginelli reaction can be catalyzed by a Brønsted acid and / or a Lewis acid such as boron trifluoride . The solid phase synthesis using various immobilized reactants is known.
Dihydropyrimidinones, the products of the Biginelli reaction, are often used as drugs .
mechanism
Using the example of the starting materials mentioned in the introduction, the reaction mechanism proceeds according to the following scheme:
Individual evidence
- ↑ P. Biginelli: Aldehyde-urea derivatives of aceto-and oxaloacetic acids. In: Gazz. Chim. Ital. 23, No. 1, 1893, pp. 360-413.
- ↑ P. Biginelli: About aldehyduramides of acetoacetic ether. In: Reports of the German Chemical Society. 24, No. 1, 1891, pp. 1317-1319, doi: 10.1002 / cber.189102401228 .
- ^ A b C. Oliver Kampe: The Biginelli Reaction . In: Jieping Zhu, Hugues Bienaymé (Ed.): Multicomponent Reactions. Wiley-VCH, Weinheim 2005, ISBN 978-3-527-30806-4 .
- ↑ Essa H. Hu, Daniel R. Sidler, Ulf-H. Dolling: Unprecedented Catalytic Three Component One-Pot Condensation Reaction: An Efficient Synthesis of 5-Alkoxycarbonyl-4-aryl-3,4-dihydropyrimidin-2 (1H) -ones. In: The Journal of Organic Chemistry. 63, No. 10, 1998, pp. 3454-3457, doi: 10.1021 / jo970846u .
- ↑ Peter Wipf, April Cunningham: A solid phase protocol of the biginelli dihydropyrimidine synthesis suitable for combinatorial chemistry. In: Tetrahedron Letters. 36, No. 43, 1995, pp. 7819-7822, doi : 10.1016 / 0040-4039 (95) 01660-A .
- ↑ C. Oliverkap : Highly versatile solid phase synthesis of biofunctional 4-aryl-3,4-dihydropyrimidines using resin-bound isothiourea building blocks and multidirectional resin cleavage. In: Bioorganic & Medicinal Chemistry Letters. 10, No. 1, 2000, pp. 49-51, doi : 10.1016 / S0960-894X (99) 00572-7 .
- ↑ George C. Rovnyak, Karnail S. Atwal, Anders Hedberg, S. David Kimball, Suzanne Moreland, Jack Z. Gougoutas, Brian C. O'Reilly, Joseph Schwartz, Mary F. Malley: Dihydropyrimidine calcium channel blockers. 4. Basic 3-substituted-4-aryl-1,4-dihydropyrimidine-5-carboxylic acid esters. Potent antihypertensive agents. In: Journal of Medicinal Chemistry. 35, No. 17, 1992, pp. 3254-3263, doi: 10.1021 / jm00095a023 .
- ^ László Kürti , Barbara Czakó: Strategic Applications of Named Reactions in Organic Synthesis . Elsevier Academic Press, Burlington / San Diego / London 2005, ISBN 0-12-369483-3 .