Bobbitt Salt
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||
| General | |||||||||||||
| Surname | Bobbitt Salt | ||||||||||||
| Molecular formula | C 11 H 21 BF 4 N 2 O 2 | ||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 336.44 g mol −1 | ||||||||||||
| Physical state |
firmly |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
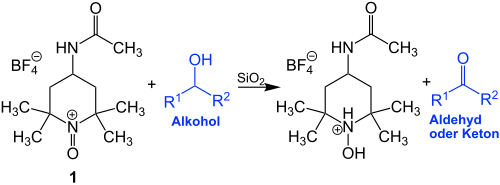
The Bobbitt salt is an oxoammonium compound, a derivative of 4-acetamido-2,2,6,6-tetramethylpiperidine. It contains a tetrafluoroborate anion and is named after its discoverer, the American chemist James M. Bobbitt (* 1930).
It is a cheaper analogue of the N -oxoammonium salt, which is derived from TEMPO . The Bobbitt Salt 1 is mainly used as a recyclable stoichiometric oxidant:
Thus, primary alcohols to aldehydes (R 1 or R 2 = H) is oxidized from secondary arise thereby alcohols ketones (R 1 , R 2 = alkyl, aryl). At the same time, 1 is reduced.
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ a b Nicholas E. Leadbeater, James M. Bobbitt: TEMPO-Derived Oxoammonium Salts as Versatile Oxidizing Agents , Aldrichimica Acta 47 (2014), pp. 65-74.
- ↑ James M Bobbitt, Nicholas A. Eddy, Jay J. Richardson, Stephanie A. Murray, Leon J. Tilley: Discussion Addendum for: Preparation of 4-Acetylamino-2, 2, 6, 6-tetramethylpiperidine-1-oxoammonium Tetrafluoroborate and the Oxidation of Geraniol to Geranial (2,6-Octadienal, 3,7-dimethyl-, (2e) -) . In: Org. Synth. . 90, 2013, p. 215. doi : 10.15227 / orgsyn.090.0215 .