Bromochlorofluoroiodomethane
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
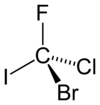
|
|||||||||||||
| General | |||||||||||||
| Surname | Bromochlorofluoroiodomethane | ||||||||||||
| Molecular formula | CIBrClF | ||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 273.27 g mol −1 | ||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Bromochlorofluoroiodomethane is a hypothetical chemical compound from the group of haloalkanes . It is a derivative of methane in which all four hydrogen atoms are replaced by one of the stable halogens . It has two enantiomers and is often cited as an example of a chiral compound.
The compound has not yet been synthesized because it is very unstable. So far only bromochlorofluoromethane could be synthesized.
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Britannica.com: Bromochlorofluoroiodomethane | chemical compound | Britannica.com , accessed October 31, 2018.
- ^ Alan R. Katritzky, Thomas L. Gilchrist, Otto Meth-Cohn, Charles Wayne Rees: Comprehensive Organic Functional Group Transformations . Elsevier, 1995, ISBN 978-0-08-042704-1 , pp. 228 ( limited preview in Google Book search).