Calcium fumarate
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
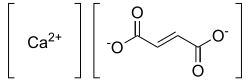
|
||||||||||
| General | ||||||||||
| Surname | Calcium fumarate | |||||||||
| Molecular formula | C 4 H 2 CaO 4 | |||||||||
| Brief description |
white solid |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 154.13 g mol −1 | |||||||||
| Physical state |
firmly |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Calcium fumarate is a chemical compound of calcium from the group of fumarates .
Extraction and presentation
Calcium fumarate trihydrate can be obtained by reacting calcium carbonate with a fumaric acid solution.
properties
Calcium fumarate and its trihydrate are white, odorless solids. The trihydrate has an orthorhombic crystal structure with the space group Pna 2 1 (space group no. 33) .
use
It is also used as an intermediate in the biochemical production of fumaric acid.
Individual evidence
- ↑ a b c jostchemical.com: Jost Chemical - Calcium Fumarate Anhydrous Purified, CAS Number 19855-56-2 , accessed on January 31, 2016
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ a b M. P. Gupta, SM Prasad, RG Sahu, BN Sahu: The crystal structure of calcium fumarate trihydrate CaC4H2O4.3H2O. In: Acta Crystallographica Section B Structural Crystallography and Crystal Chemistry. 28, p. 135, doi : 10.1107 / S0567740872001967 .
- ↑ Mira Madan, KS Thind: Physiology of Fungi . APH Publishing, 1998, ISBN 978-81-7024-941-2 , pp. 134 ( limited preview in Google Book search).