Carbonyl bromide
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
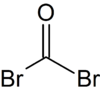
|
||||||||||
| General | ||||||||||
| Surname | Carbonyl bromide | |||||||||
| other names |
|
|||||||||
| Molecular formula | CBr 2 O | |||||||||
| Brief description |
colorless liquid with a strong odor |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 187.81 g mol −1 | |||||||||
| Physical state |
liquid |
|||||||||
| density |
2.5 g cm −3 |
|||||||||
| boiling point |
65 ° C |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Carbonyl bromide ( bromophosgene ) is a chemical compound from the group of carbonyl compounds .
Extraction and presentation
Carbonyl bromide was first synthesized in 1863 by J. Schiel by reacting carbon monoxide with bromine vapor in sunlight.
Today it is obtained by reacting carbon tetrabromide with sulfuric acid at 150–170 ° C with a yield of about 50%.
properties
Carbonyl bromide is a colorless liquid with a strong odor that smokes in air and decomposes in light and when heated. It hydrolyzes in water to form hydrogen bromide and carbon dioxide .
use
Carbonyl bromide is used to make dyes.
Individual evidence
- ↑ a b c d e f Dale L. Perry: Handbook of Inorganic Compounds . CRC Press, 1995, ISBN 978-0-8493-8671-8 , pp. 101 ( limited preview in Google Book search).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ a b T.A. Ryan, EA Seddon, KR Seddon, C. Ryan: Phosgene And Related Carbonyl Halides . Elsevier, 1996, ISBN 978-0-08-053880-8 , pp. 666–667 ( limited preview in Google Book Search).
![{\ displaystyle \ mathrm {CBr_ {4} \ + \ H_ {2} SO_ {4} \ {\ xrightarrow [{}] {}}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/2a8a5bef178ae5bdc3cf1ba16c43ef54a28808f3)
![{\ displaystyle \ mathrm {\ COBr_ {2} + \ 2HBr + \ SO_ {3} \ {\ xrightarrow [{}] {}}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/63ed12afaa8b658d55d75e8bc448ae6d1dccb235)
