Cerium (III) oxalate
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||
| General | ||||||||||
| Surname | Cerium (III) oxalate | |||||||||
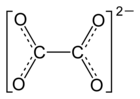
| Molecular formula |
|
|||||||||
| Brief description |
white solid |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| Drug information | ||||||||||
| ATC code | ||||||||||
| properties | ||||||||||
| Molar mass |
|
|||||||||
| Physical state |
firmly |
|||||||||
| solubility |
bad in water |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Cerium (III) oxalate is a compound of the rare earth metal cerium with oxalic acid . It is a white crystalline powder and is insoluble in water and organic solvents.
presentation
The representation succeeds by reacting oxalic acid with cerium (III) chloride ; the latter is prepared from cerium-containing flints and hydrochloric acid . The insoluble product is obtained as a crystalline precipitate .
Reactions
Cer (III) oxalate decomposes when heated from approx. 275 ° C to the cerium oxides cerium (III) oxide and cerium (IV) oxide .
Individual evidence
- ↑ a b Data sheet Cerium (III) oxalate nonahydrate (PDF) from Strem, accessed on December 25, 2012.
- ↑ a b data sheet cerium (III) oxalate from Sigma-Aldrich , accessed on November 1, 2016 ( PDF ).
- ^ S. El-Houte, M. El-Sayed Ali: Thermal decomposition of cerium oxalate and mixed cerium-gadolinium oxalates . In: Journal of Thermal Analysis . tape 37 , 1991, pp. 907-913 , doi : 10.1007 / BF01932788 .

