Chemistry of carbon nanotubes
The chemistry of carbon nanotubes ( English carbon nanotube chemistry ) deals with chemical reactions that are used to change the properties of carbon nanotubes (CNTs). CNTs can be functionalized in such a way that they have the desired properties for a wide range of applications.
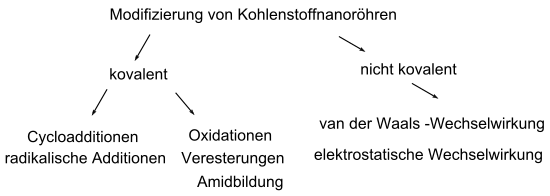
Two important methods are the functionalization of covalent and non-covalent bonds.
Because of their hydrophobic properties, CNTs tend to clump together, which prevents dispersion in solvents or in viscous polymer melts. The resulting nanotube bundles or coils make the final material less useful. The surface of the CNTs can now be varied in such a way that they are less hydrophobic and that the surface adhesion to a polymer is improved by attaching functional groups .
Toxicity of the functionalized CNTs
It can be assumed that functionalized CNTs are cytotoxic.
Covalent modification
In covalent modification, a functional group is attached to a nanotube. A functional group can be attached either to the side wall or to the end of a nanotube. The ends of the nanotube have the greatest reactivity because of their larger pyramidalization angle, and the side walls of the carbon nanotube have a smaller pyramidalization angle, which has a lower reactivity. Although covalent modifications are very stable, the sp 2 hybridization of the carbon atom to which one is attached is broken because a new σ-bond is formed. The dissolution of the delocalized π-electron system typically reduces the conductivity of the carbon nanotube.
oxidation
The purification and oxidation of CNTs has often been mentioned in the literature. These processes were important for a low-yield representation of carbon nanotubes, where carbon particles and amorphous carbon make up a significant proportion of the material and are still important in attaching functional groups to the surface. During the oxidation, the carbon-carbon bond network of the graphite layers is broken by allowing the introduction of oxygen units, thus forming carboxyl, phenol and lactone groups, which have been sufficiently explored for further chemical functionalization.
Initial investigations into the oxidation of nanotubes used a gas phase reaction with nitric acid vapor, whereby the nanotubes were unselectively functionalized with carboxyl, carbonyl and hydroxyl groups. In liquid phase reactions, nanotubes were treated with the oxidizing solutions nitric acid or aqua regia with the same effect. However, "over-oxidation" can cause the nanotube to break apart into fragments known as carbon fragments. Xing et al. investigated ultrasound-assisted oxidation of CNTs with sulfuric and nitric acid and produced carbonyl and carboxyl groups. If the oxidation with acid is stopped by treatment with hydrogen peroxide, the destruction of the CNT is prevented. Single-walled nanotubes can be shortened with oleum (100% H 2 SO 4 with 3% SO 3 ) and nitric acid.
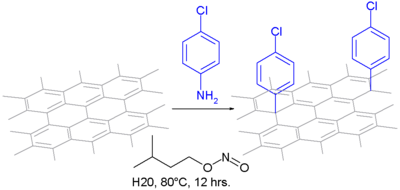
In one type of reaction, it is oxidized with aniline to the diazonium salt and flushed with nitrogen, so that a covalent bond is formed in which an aryl radical is formed. The oxidation reagent is isoamyl nitrite in an aqueous suspension.
Esterification / amide formation
Carboxylic acids are the precursors for esterification and amide formation. The carboxyl group is first converted to the acid chloride with thionyl chloride or with oxalyl chloride, which is then converted to the amide, amine or alcohol. CNTs can be coated with silver nano-particles using amination reactions. Amide-functionalized CNTs were presented as chelates for silver nanoparticles. CNTs modified with acyl chloride react with branched molecules such as polyamidoamine, which can be used as a template for silver ions and is later reduced with formamide . Amino-modified CNTs can be prepared by reacting an acyl chloride-modified CNT with ethylenediamine .
Cycloaddition
There are cycloaddition reactions such as Diels-Alder reactions or 1,3-dipolar cycloaddition with azomethine ylidene known, as azide-alkyne cycloaddition. An example is a Diels-Alder reaction with chromium hexacarbonyl under high pressure. The I D / I G isomer ratio in the reaction with Danishefsky ’s diene is 2.6.
The best-known 1,3-cycloaddition is the reaction of a CNT with an azomethine ylide. The addition of pyrrolidine can lead to a number of functional groups, such as polyamidoamine dendrimers , phthalocyanines, perfluoroalkylsilanes, and aminoethylene glycol groups. The Diels – Alder cycloaddition occurs primarily with fluorinated CNTs. As is known, starting materials for Diels-Alder reactions are dienes such as 2,3-dimethyl-1,3-butadienes, anthracenes and 2-trimethylsiloxyl-1,3-butadienes.
Radical addition
The modification of CNTs with aryldiazonium salts was first described by Tour et al. examined. Because of the extreme reaction conditions for the in situ generation of the diazonium ion, other methods were sought. Stephenson et al. report a reaction of aniline derivatives with sodium nitride in 96% sulfuric acid and ammonium persulfate. Price et al. conducted the reaction under milder reaction conditions by stirring CNTs in water and treating them with aniline and mild oxidizing agent. Diazonium chemistry has been used to make further modifications to CNTs such as: B. with the Suzuki reaction and the Heck reaction . Coupling reactions were performed with iodophenyl-functionalized CNTs. Wong et al. showed silylation reactions with trimethoxysilane and with heptaphenyldisilane under mild photochemical reaction conditions.
Nucleophilic addition
Hirsch et al. performed nucleophilic additions with organolithium reagents and with organomagnesium compounds to CNTs. After subsequent air oxidation, they were able to produce alkyl-modified CNTs. Hirsch was able to demonstrate nucleophilic addition with amines by generating lithium amides, which led to amino-modified CNTs.
Electrophilic addition
Nanotubes can also be alkylated with alkyl halides using lithium or sodium and liquid ammonia, as in a Birch reduction . Nanotube salts can act as a polymerization initiator and can react with peroxides to form alkoxy-functionalized nanotubes.
The alkyl and hydroxy modification of CNTs was performed by the electrophilic addition of alkyl halides under microwave irradiation. Tessonier et al. modified CNTs by deprotonation with butyllithium and subsequent amino substitution. Balaban et al. performed Friedel-Crafts alkylations with nitrobenzene at 180 ° C with aluminum chloride on CNTs.
Non-covalent modifications
Non-covalent modifications use van der Waals forces and π-π interactions through adsorption of polycondensed aromatics, surfactants , polymers and biomolecules. Non-covalent modifications do not destroy the configuration of the CNT at the expense of chemical stability and are advantageous for phase separations and dissociations of solids between two phases.
Condensed aromatic compounds
Condensed aromatics, which have hydrophilic and hydrophobic units, are used to dissolve CNTs in organic or aqueous solutions. Some of these amphiphiles are phenyl , naphthyl, phenanthryl, pyrene , and porphyrin systems. The greater the π-π interaction of the aromatic amphiphiles, the better the solubility. These aromatic systems can be varied with amino and carboxyl groups to functionalize the CNTs.
Biomolecules
The interactions between CNTs and biomolecules have been extensively studied because of their potential for biological applications. Modifications of CNTs have been visualized with proteins, carbohydrates and nucleic acids using the bottom-up technique . Proteins have a high affinity for CNTs because the amino acids they are made up of can be both hydophilic and hydophobic. Polysaccharides have been used successfully to modify CNTs to form stable hybrids. To make CNTS water soluble, phospholipids such as lysoglycerophosopholipids have been used.
π-π interaction and electrostatic interactions
Bifunctional molecules are used to modify CNTs. One end of the molecule is a polyaromatic component that enters into π-π interactions with the nanotube . The other end of the same molecule has a functional group such as amino, carboxy or thiol groups. For example, pyrene derivatives and aryl thiols have been used as substrates for various metal nanobeads such as gold, silver and platinum.
characterization
A useful analytical method for CNTs is Raman spectroscopy . There is a G-band at 1580 cm −1 and a D-band at 1350 cm −1 . The ratio of the two peaks indicates the degree of functionalization.
literature
- Klaus Müllen , Xinliang Feng (Ed.): Chemistry of Carbon-Nanostructures , De Gruyter 2017
Individual evidence
- ↑ a b c d e f g h i Nikolaos Karousis, Nikos Tagmatarchis, Dimitrios Tasis: Current Progress on the Chemical Modification of Carbon Nanotubes . In: Chemical Reviews . 110, No. 9, June 14, 2010, pp. 5366-5397. doi : 10.1021 / cr100018g . PMID 20545303 .
- ↑ Prem Kumarathasan, Dalibor Breznan, Dharani Das, Mohamed A. Salam, Yunus Siddiqui, Christine MacKinnon-Roy, Jingwen Guan, Nimal de Silva, Benoit Simard, Renaud Vincent: Cytotoxicity of carbon nanotube variants: A comparative in vitro exposure study with A549 epithelial and J774 macrophage cells. In: Nanotoxicology . 9 (2), 2014, pp. 148-161, doi: 10.3109 / 17435390.2014.902519 .
- ↑ SC Tsang, PJF Harris, MLH Green: thinning and opening of carbon nanotubes by oxidation using carbon dioxide . In: Nature . 362, No. 6420, 1993, pp. 520-522. doi : 10.1038 / 362520a0 .
- ^ PM Ajayan, TW Ebbesen, T. Ichihashi, S. Iijima, K. Tanigaki, H. Hiura: Opening carbon nanotubes with oxygen and implications for filling . In: Nature . 362, No. 6420, 1993, pp. 522-525. doi : 10.1038 / 362522a0 .
- ↑ SC Tsang, YK Chen, PJF Harris, MLH Green: A simple chemical method of opening and filling carbon nanotubes . In: Nature . 372, No. 6502, 1994, pp. 159-162. doi : 10.1038 / 372159a0 .
- ↑ Hidefumi Hiura, Thomas W. Ebbesen, Katsumi Tanigaki: Opening and purification of carbon nanotubes in high yields . In: Advanced Materials . 7, No. 3, 1995, pp. 275-276. doi : 10.1002 / adma.19950070304 .
- ↑ K Esumi, M. Ishigami, A. Nakajima, K. Sawada, H. Honda: Chemical treatment of carbon nanotubes . In: Carbon . 34, No. 2, 1996, pp. 279-281. doi : 10.1016 / 0008-6223 (96) 83349-5 .
- ↑ M Shaffer, X. Fan, AH Windle: Dispersion and packing of carbon nanotubes . In: Carbon . 36, No. 11, 1998, pp. 1603-1612. doi : 10.1016 / S0008-6223 (98) 00130-4 .
- ↑ Ya-Ping Sun, Kefu Fu, Yi Lin, Weijie Huang: Functionalized Carbon Nanotubes: Properties and Applications . In: Accounts of Chemical Research . 35, No. 12, 2002, pp. 1096-104. doi : 10.1021 / ar010160v . PMID 12484798 .
- ↑ Wei Xia, Chen Jin, Shankhamala Kundu, Martin Muhler: A highly efficient gas-phase route for the oxygen functionalization of carbon nanotubes based on nitric acid vapor . In: Carbon . 47, No. 3, March 1, 2009, pp. 919-922. doi : 10.1016 / j.carbon.2008.12.026 .
- ↑ V. Datsyuk, M. Kalyva, K. Papagelis, J. Parthenios, D. Tasis, A. Siokou, I. Kallitsis, C. Galiotis: Chemical oxidation of multiwalled carbon nanotubes . In: Carbon . 46, No. 6, May 1, 2008, pp. 833-840. doi : 10.1016 / j.carbon.2008.02.012 .
- ↑ Céline Bergeret, Jack Cousseau, Vincent Fernandez, Jean-Yves Mevellec, Serge Lefrant: Spectroscopic Evidence of Carbon Nanotubes' Metallic Character Loss Induced by Covalent Functionalization via Nitric Acid Purification . In: Journal of Physical Chemistry C . 112, No. 42, October 23, 2008, pp. 16411-16416. doi : 10.1021 / jp806602t .
- ↑ Yangchuan Xing, Liang Li, Charles C. Chusuei, Robert V. Hull: Sonochemical Oxidation of Multiwalled Carbon Nanotubes . In: Langmuir . 21, No. 9, April 1, 2005, pp. 4185-4190. doi : 10.1021 / la047268e .
- ↑ F. Avilés, JV Cauich-Rodríguez, L. Moo-Tah, A. May-Pat, R. Vargas-Coronado: Evaluation of mild acid oxidation treatments for MWCNT functionalization . In: Carbon . 47, No. 13, November 1, 2009, pp. 2970-2975. doi : 10.1016 / j.carbon.2009.06.044 .
- ^ BK Price, JM Tour: Functionalization of Single-Walled Carbon Nanotubes "On Water" . In: Journal of the American Chemical Society . 128, No. 39, 2006, pp. 12899-12904. doi : 10.1021 / ja063609u . PMID 17002385 .
- ↑ Nikolaos Karousis, Nikos Tagmatarchis, Dimitrios Tasis: Current Progress on the Chemical Modification of Carbon Nanotubes . In: Chemical Reviews . 110, No. 9, June 14, 2010, pp. 5366-5397. doi : 10.1021 / cr100018g . PMID 20545303 .
- ↑ Lei Tao, Chen Gaojian, Giuseppe Mantovani, Steve York, David M. Haddleton: Modification of multi-wall carbon nanotube surfaces with poly (amidoamines) dendrons . In: Synthesis and metal templating . No. 47, September, p. 4949. doi : 10.1039 / B609065F .
- ↑ a b J. S. Jeong, SY Jeon, TY Lee, JH Park, JH Shin, PS Alegaonkar, AS Berdinsky, JB Yoo: Fabrication of MWNTs / nylon conductive composite nanofibers by electrospinning . In: Diamond and Related Materials . 15, No. 11-12, November 1, 2006, pp. 1839-1843. Proceedings of the joint 11th International Conference on New Diamond Science and Technology and the 9th Applied Diamond Conference 2006. doi : 10.1016 / j.diamond.2006.08.026 .
- ^ I. Kumar, S. Rana, JW Cho: Cycloaddition Reactions: A Controlled Approach for Carbon Nanotube Functionalization . In: Chemistry: A European Journal . 17, No. 40, 2011, pp. 11092-11101. doi : 10.1002 / chem.201101260 .
- ↑ CC Ménard-Moyon, FO Dumas, E. Doris, C. Mioskowski: Functionalization of Single-Wall Carbon Nanotubes by Tandem High-Pressure / Cr (CO) 6 Activation of Diels − Alder Cycloaddition . In: Journal of the American Chemical Society . 128, No. 46, 2006, pp. 14764-14765. doi : 10.1021 / ja065698g . PMID 17105260 .
- ↑ Stéphane Campidelli, Chloé Sooambar, Enrique Lozano Diz, Christian Ehli, Dirk M. Guldi, Maurizio Prato: Dendrimer-Functionalized Single-Wall Carbon Nanotubes: Synthesis, Characterization, and Photoinduced Electron Transfer . In: Journal of the American Chemical Society . 128, No. 38, September 1, 2006, pp. 12544-12552. doi : 10.1021 / ja063697i . PMID 16984205 .
- ↑ Beatriz Ballesteros, Gema de la Torre, Christian Ehli, GM Aminur Rahman, F. Agulló-Rueda, Dirk M. Guldi, Tomás Torres: Single-Wall Carbon Nanotubes Bearing Covalently Linked Phthalocyanines - Photoinduced Electron Transfer . In: Journal of the American Chemical Society . 129, No. 16, April 1, 2007, pp. 5061-5068. doi : 10.1021 / ja068240n . PMID 17397152 .
- ↑ Vasilios Georgakilas, Athanasios B. Bourlinos, Radek Zboril, Christos Trapalis: Synthesis, Characterization and Aspects of Superhydrophobic Functionalized Carbon Nanotubes . In: Chemistry of Materials . 20, No. 9, May 1, 2008, pp. 2884-2886. doi : 10.1021 / cm7034079 .
- ↑ Bruno Fabre, Fanny Hauquier, Cyril Herrier, Giorgia Pastorin, Wei Wu, Alberto Bianco, Maurizio Prato, Philippe Hapiot, Dodzi Zigah: Covalent Assembly and Micropatterning of Functionalized Multiwalled Carbon Nanotubes to Monolayer-Modified Si (111) Surfaces . In: Langmuir . 24, No. 13, July 1, 2008, pp. 6595-6602. doi : 10.1021 / la800358w .
- Jump up ↑ T Umeyama, J Baek, Y Sato, K Suenaga, F Abou-Chahine, NV Tkachenko, H Lemmetyinen, H Imahori: Molecular interactions on single-walled carbon nanotubes revealed by high-resolution transmission microscopy . In: Nature Communications . 6, 2015, p. 7732. doi : 10.1038 / ncomms8732 . PMID 26173983 . PMC 4518305 (free full text).
- ^ Hugh Hayden, Yurii K. Gun'ko, Tatiana S. Perova: Chemical modification of multi-walled carbon nanotubes using a tetrazine derivative . In: Chemical Physics Letters . 435, No. 1-3, February 12, 2007, pp. 84-89. doi : 10.1016 / j.cplett.2006.12.035 .
- ↑ Jason J. Stephenson, Jared L. Hudson, Samina Azad, James M. Tour: Individualized Single Walled Carbon Nanotubes from Bulk Material Using 96% Sulfuric Acid as Solvent . In: Chemistry of Materials . 18, No. 2, January 1, 2006, pp. 374-377. doi : 10.1021 / cm052204q .
- ↑ Fuyong Cheng, Patigul Imin, Christian Maunders, Gianluigi Botton, Alex Adronov: Soluble, Discrete Supramolecular Complexes of Single-Walled Carbon Nanotubes with Fluorene-Based Conjugated Polymers . In: Macromolecules . 41, No. 7, March 4, 2008, pp. 2304-2308. doi : 10.1021 / ma702567y .
- ↑ Roberto Martín, Liliana Jiménez, Mercedes Alvaro, Juan C. Scaiano, Hermenegildo Garcia: Two-Photon Chemistry in Ruthenium 2,2′-Bipyridyl-Functionalized Single-Wall Carbon Nanotubes . In: Chemistry: A European Journal . 16, No. 24, June 25, 2010, pp. 7282-7292. doi : 10.1002 / chem.200903506 .
- ↑ Ralf Graupner, Jürgen Abraham, David Wunderlich, Andrea Vencelová, Peter Lauffer, Jonas Röhrl, Martin Hundhausen, Lothar Ley, Andreas Hirsch: Nucleophilic − Alkylation − Reoxidation: A Functionalization Sequence for Single-Wall Carbon Nanotubes . In: Journal of the American Chemical Society . 128, No. 20, May 1, 2006, pp. 6683-6689. doi : 10.1021 / ja0607281 . PMID 16704270 .
- ↑ a b Zois Syrgiannis, Frank Hauke, Jonas Röhrl, Martin Hundhausen, Ralf Graupner, Yiannis Elemes, Andreas Hirsch: Covalent Sidewall Functionalization of SWNTs by Nucleophilic Addition of Lithium Amides . In: European Journal of Organic Chemistry . 2008, No. 15, May 1, 2008, pp. 2544-2550. doi : 10.1002 / ejoc.200800005 .
- ↑ F. Liang, AK Sadana, A. Peera, J. Chattopadhyay, Z. Gu, RH Hauge, WE Billups: A Convenient Route to Functionalized Carbon Nanotubes . In: Nano Letters . 4, No. 7, 2004, pp. 1257-1260. bibcode : 2004NanoL ... 4.1257L . doi : 10.1021 / nl049428c .
- ↑ D. Wunderlich, F. Hauke, A. Hirsch: Preferred functionalization of metallic and small-diameter single walled carbon nanotubes via reductive alkylation . In: Journal of Materials Chemistry . 18, No. 13, 2008, p. 1493. doi : 10.1039 / b716732f .
- ↑ F. Liang, JM Beach, K. Kobashi, AK Sadana, YI Vega-Cantu, JM Tour, WE Billups: In Situ Polymerization Initiated by Single-Walled Carbon Nanotube Salts . In: Chemistry of Materials . 18, No. 20, 2006, pp. 4764-4767. doi : 10.1021 / cm0607536 .
- ↑ A. Mukherjee, R. Combs, J. Chattopadhyay, DW Abmayr, PS Engel, WE Billups: Attachment of Nitrogen and Oxygen Centered Radicals to Single-Walled Carbon Nanotube Salts . In: Chemistry of Materials . 20, No. 23, 2008, pp. 7339-7343. doi : 10.1021 / cm8014226 .
- ↑ TS Balaban, MC Balaban, S. Malik, F. Hennrich, R. Fischer, H. Rösner, MM Kappes: Polyacylation of Single-Walled Nanotubes under Friedel-Crafts Conditions: An Efficient Method for Functionalizing, Purifying, Decorating, and Linking Carbon allotropes . In: Advanced Materials . 18, No. 20, October 17, 2006, pp. 2763-2767. doi : 10.1002 / adma.200600138 .
- ↑ a b Yasuhiko Tomonari, Hiroto Murakami, Naotoshi Nakashima: Solubilization of Single-Walled Carbon Nanotubes by using Polycyclic Aromatic Ammonium Amphiphiles in Water — Strategy for the Design of High-Performance Solubilizers . In: Chemistry: A European Journal . 12, No. 15, May 15, 2006, pp. 4027-4034. doi : 10.1002 / chem.200501176 .
- ↑ Trevor J. Simmons, Justin Bult, Daniel P. Hashim, Robert J. Linhardt, Pulickel M. Ajayan: Noncovalent Functionalization as an Alternative to Oxidative Acid Treatment of Single Wall Carbon Nanotubes with Applications for Polymer Composites . In: ACS Nano . 3, No. 4, April 28, 2009, pp. 865-870. doi : 10.1021 / nn800860m .
- ↑ Wenrong Yang, Pall Thordarson, J Justin Gooding, Simon P Ringer, Filip Braet: Carbon nanotubes for biological and biomedical applications . In: Nanotechnology . 18, October 17, 2007, p. 412001. doi : 10.1088 / 0957-4484 / 18/41/412001 .
- ↑ Hui Yang, Shiunchin C. Wang, Philippe Mercier, Daniel L. Akins: Diameter-selective dispersion of single-walled carbon nanotubes using a water-soluble, biocompatible polymer . In: Chemical Communications . No. 13, September, p. 1425. doi : 10.1039 / B515896F .
- ↑ Ran Chen, Slaven Radic, Poonam Choudhary, Kimberley G. Ledwell, George Huang, Jared M. Brown, Pu Chun Ke: Formation and cell translocation of carbon nanotube-fibrinogen protein corona . In: Applied Physics Letters . 101, No. 13, September 24, 2012, p. 133702. doi : 10.1063 / 1.4756794 . PMID 23093808 . PMC 3470598 (free full text).
- ↑ Zhijuan Wang, Meiye Li, Yuanjian Zhang, Junhua Yuan, Yanfei Shen, Li Niu, Ari Ivaska: Thionine-interlinked multi-walled carbon nanotube / gold nanoparticle composites . In: Carbon . 45, No. 10, September 1, 2007, pp. 2111-2115. doi : 10.1016 / j.carbon.2007.05.018 .