Dimethyl magnesium
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
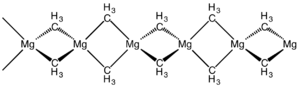
|
||||||||||
| General | ||||||||||
| Surname | Dimethyl magnesium | |||||||||
| other names |
Magnesium dimethyl |
|||||||||
| Molecular formula | C 2 H 6 Mg | |||||||||
| Brief description |
white solid |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 54.37 g mol −1 | |||||||||
| Physical state |
firmly |
|||||||||
| Melting point |
250 ° C (decomposition) |
|||||||||
| solubility |
practically insoluble in hydrocarbons |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Dimethylmagnesium is a chemical compound from the group of organomagnesium compounds .
Extraction and presentation
Dimethylmagnesium, like other dialkylmagnesium compounds, can be obtained by adding dioxane to a solution of a methylmagnesium halide.
It can also be made by reacting calcium , magnesium and methyl iodide in diethyl ether or by reacting dimethylmercury with magnesium.
properties
Dimethylmagnesium is a white polymeric solid that is practically insoluble in hydrocarbons. It ignites on contact with water. Like dimethylberyllium it forms high molecular chains in an orthorhombic crystal structure (a = 6.00; b = 11.48; c = 5.45 Å) with the probable space group Ibam (space group no. 72) .
Individual evidence
- ↑ a b c d e Asutosh Samantaray, Balaram Sahoo, Nimai Charan Nayak, Prafulla Kumar: Inorganic Chemistry . PHI Learning Pvt. Ltd., 2012, ISBN 978-81-203-4308-5 , pp. 203 ( limited preview in Google Book search).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Arthur C. Cope: The Preparation of Dialkylmagnesium Compounds from Grignard Reagents. In: Journal of the American Chemical Society. 57, 1935, p. 2238, doi : 10.1021 / ja01314a059 .
- ↑ Houben-Weyl Methods of Organic Chemistry Vol. XIII / 2a, 4th Edition Organometallic Compounds of Group II of the Periodic Table (except mercury) . Georg Thieme Verlag, 2014, ISBN 3-13-180654-0 , p. 215 ( limited preview in Google Book search).
- ↑ Jane E. Macintyre: Dictionary of Organometallic Compounds . CRC Press, 1994, ISBN 978-0-412-43060-2 , pp. 2273 ( limited preview in Google Book search).
- ↑ Peter Urben: Bretherick's Handbook of Reactive Chemical Hazards . Academic Press, 2013, ISBN 0-08-052340-4 , pp. 2166 ( limited preview in Google Book search).
- ↑ E. Weiss: The crystal structure of dimethyl magnesium. In: Journal of Organometallic Chemistry. 2, 1964, p. 314, doi : 10.1016 / S0022-328X (00) 82217-2 .