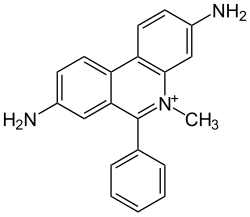
Dimidium bromide
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
 |
||||||||||
| General | ||||||||||
| Surname | Dimidium bromide | |||||||||
| other names |
|
|||||||||
| Molecular formula | C 20 H 18 BrN 3 | |||||||||
| Brief description |
red-brown odorless solid |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 380.28 g mol −1 | |||||||||
| Physical state |
firmly |
|||||||||
| Melting point |
243-248 ° C |
|||||||||
| solubility |
soluble in water (at 20 ° C) |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| Toxicological data | ||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Dimidium bromide is a polycyclic organic compound from the group of phenanthridine dyes . The red, odorless substance is sparingly soluble in water, better in organic solvents such as chloroform, dichloromethane and methanol. The homologous ethyl derivative is ethidium bromide .
use
Dimidium bromide is used together with disulfine blue VN 150 as a mixed indicator for an Epton titration . It is also used as a detection reagent for nucleic acids .
Individual evidence
- ↑ a b c data sheet dimidium bromide (PDF) from Merck , accessed on December 5, 2016.
- ↑ a b c Datasheet Dimidium bromide from Sigma-Aldrich , accessed on December 5, 2016 ( PDF ).
- ↑ a b data sheet ( page no longer available , search in web archives ) at caledonlabs.com (PDF; 53 kB).
- ↑ G. Dougherty: A comparison of the base-pair specificities of three phenanthridine drugs using solution spectroscopy , in: Int. J. Biochem. 1982 , 14 (6) , pp. 493-504; PMID 7106349 .
