Dolasetron
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
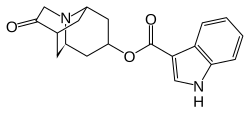
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Dolasetron | |||||||||||||||||||||
| other names |
(3 R ) -10-Oxo-8-azatricyclo [5.3.1.0 3,8 ] undec-5-yl-1 H -indole-3-carboxylate ( IUPAC ) |
|||||||||||||||||||||
| Molecular formula | C 19 H 20 N 2 O 3 | |||||||||||||||||||||
| Brief description |
white powder (mesylate hydrate) |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 324.37 g · mol -1 | |||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Dolasetron is a 5-HT3 antagonist used to treat nausea and vomiting in chemotherapy and PONV . Dolasetron is administered in a dosage of 50 to 200 milligrams. The half-life is 7 to 9 hours. Another area of application of Dolasetron is veterinary medicine , where it is also used against vomiting.
Side effects
Its most common side effects are: severe allergic reactions, fast or pounding heartbeats, fainting or fainting , slow heartbeats, weak pulse, slow breathing, chest pain, prolongation of the QT / QTc interval , pain in the arm or leg, nausea, sweating , general mood, headache, tiredness.
Trade names
Dolasetron came onto the German market in 1997 under the trade name Anemet . Approvals for all dosage forms were given up by the manufacturer in 2011 after cardiac arrhythmias occurred as a side effect of intravenous administration .
Individual evidence
- ↑ a b Data sheet Dolasetron mesylate hydrate, ≥98% (HPLC), powder from Sigma-Aldrich , accessed on January 25, 2013 ( PDF ).
- ↑ Josef Donnerer: Antiemetic Therapy. ISBN 3-8055-7547-5 .
- ↑ Anzemet Injection (Dolasetron) - Description and Clinical Pharmacology. In: DrugLib.com. 2012, accessed January 22, 2013 .
- ↑ Harald Schmidt: Pharmacology and Toxicology. ISBN 3-7945-2295-8 .
- ↑ Reto Neiger: Differential Diagnoses of Internal Medicine in Dogs and Cats: From Key Symptom to Diagnosis . Georg Thieme Verlag, 2009, ISBN 978-3-8304-1119-2 .
- ↑ Anzemet Tablets (Dolasetron) Drug Information: Description, User Reviews, Drug Side Effects, Interactions - Prescribing Information at RxList. RxList Inc., June 10, 2011, accessed January 22, 2013 .
- ↑ Side effects of Dolasetron - for the consumer. In: Side Effects.co. 2013, archived from the original on March 4, 2016 ; Retrieved January 22, 2013 .
- ↑ Information letter for medical professionals on Anemet 200 mg tablets. (PDF; 138 kB) Sanofi-Aventis Deutschland GmbH, April 2001, accessed on January 22, 2013 .