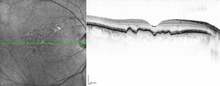
Druse (retina)
| Classification according to ICD-10 | |
|---|---|
| H35.3 | Degeneration of the macula and posterior pole |
| ICD-10 online (WHO version 2019) | |
As drusen is known deposits of extracellular material beneath the retina. They occur ubiquitously in all age groups, but increase in size and number with increasing age. They are considered to be the early form of age-related macular degeneration . Drusen do not cause visual impairment per se, but can lead to disturbances in color and contrast sensitivity.
Etymology and history
Druze was first described by FC Donders in 1855, in the first volume of the Archives of Ophthalmology . He discovered spherical deposits beneath the retina of an 80-year-old patient and dissected them. Based on the experiments he carried out, he came to the conclusion that they could best be compared to colloidal tissue:
But what is it to be called? At the moment I do not really know, but the concept of the hybrid word colloid is so flexible that I do not think I am indiscreet if I ask for a place in it for these balls for the time being. So I just want to call them colloid balls.
And further:
How and from what do these spheres develop? At first sight it already seemed to me that they arise from the nuclei of the pigment cells . Further investigations have completely convinced me of the correctness of this assumption.
This finding laid the foundation for the transformation theory about the formation of Druze. Just one year later, in 1856, H. Müller developed the so-called deposition theory , according to which drusen arise from deposits of breakdown products in the retina. He also introduced the term "druse" based on its appearance, which reminded him of the mineral rock formations . The term druse finally established itself in the international specialist literature.
Definition and forms
By definition, a druse is a focal deposit of extracellular material between the basal lamina of the retinal pigment epithelium and the inner collagenous layer of Bruch's membrane .
Donders described the different appearance of drusen in his scientific essay. Clinically today, a distinction is made between hard and soft drusen, depending on their margins, as well as calcified drusen, if these have a highly reflective whitish content under fundoscopy . Histologically , however, a much greater variety of different drusen shapes was observed. A similar variety can be found in the tomography sections of optical coherence tomography .
A more precise assessment of the clinical significance of these different types of glands for the progression of age-related macular degeneration is still pending.
Epidemiology
98.8% of the total population have at least one druse. Small hard drusen (less than 63 µm in diameter) are by far the most common, which suggests that these are physiological phenomena without disease value. The prevalence of drusen over 63 µm in diameter and soft drusen increases with age; they can be found in around 60% of all people over the age of 75.
Emergence
The exact mechanism of formation of drusen is still largely unknown despite numerous scientific research work. In general, a mixture of the above-mentioned transformation and deposition theory is assumed. This z. B. pigment epithelial cells damaged by oxidative stress and a corresponding genetic predisposition as a trigger for immune-mediated processes, in which the complement system seems to play a major role. Subsequently, immune complexes , proteins and lipids are deposited .
Druze components
The main component of drusen are lipids, especially cholesterol , which is also responsible for the yellowish appearance of drusen. There are also carbohydrates , zinc and extracellular matrix components . To date, at least 129 different proteins have been identified inside the gland, the majority of which are related to inflammation or immune-associated processes. In addition, various cell components, such as lipofuscin and melanin , up to complete cells such as dendritic cells can be found in drusen .
Further course
On the basis of the histological studies, one can only speculate about the further development of drusen. In the physiological form of drusen, it is assumed that after the pigment epithelium has healed, the deposits are channeled through the Bruch's membrane and absorbed by the choroid . The druse disappears without a trace after a while. However, especially in the soft drusen observed in old age, there seems to be irreversible damage to the pigment epithelium, which is further promoted by the development of a druse. In addition, the removal of the glandular components should be significantly reduced by an age-related thickened Bruch's membrane. Finally, there is complete degeneration and atrophy of the pigment epithelium over the gland and, as a result, degeneration of the photoreceptors above . By definition, this stage marks the beginning of age-related macular degeneration (AMD).
Drusen as a risk factor for age-related macular degeneration
The appearance of soft drusen with or without pigment shifts characterizes the clinical picture of age-related maculopathy by definition . Hard drusen are currently not considered to be a disease. Age-related maculopathy is often referred to as the "early form" of AMD. Based on these classifications, which flow into AMD, the large-scale, multicenter Age-Related Eye Disease Study (AREDS) was set up in the 1990s, with the aim of investigating the clinical phenomena and developments in age-related maculopathy and degeneration to investigate. The number of drusen and their total surface area per eye could be established as a clinically significant risk factor for the development of AMD. However, the estimates differ as to the exact risk potential. Studies report a risk of developing wet AMD within the next 5 years with bilateral drusen between 0.2% and 40%. These large differences suggest that there are a number of different types of gland for which appropriate study models would have to be developed.
clinic
Druze cause per se no disturbances of vision and therefore are usually an incidental finding. In some cases, however, with mostly a large number of drusen, disturbances in contrast vision and color perception and a decrease in sensitivity in the central visual field are reported.
Drusen in diseases other than AMD
Drusen are primarily understood as an early form of AMD, but they can also be found in other diseases, such as above pigmented nevi or malignant melanomas . Drusen in long-standing central serous retinopathy have also been described. In addition, a connection between drusen and glomerulonephritis type II was found, which is interesting in that it is most likely the same mechanism underlying pathological deposits. Furthermore, drusen are found in some familial macular degenerations, such as Malattia Leventinese (also Doyn's honeycomb dystrophy ) and Sorsby fundic dystrophy .
therapy
According to the current state of medical science, there is no effective therapy for drusen. The laser coagulation therapy, which was popular in the 90s, was able to show an impressive regression of soft drusen after laser coagulation , but the risk of developing AMD did not decrease. According to the results of the AREDS study, prophylactic vitamin supplementation with lutein and omega-3 fatty acids is recommended for patients with drusen . This significantly lowers the risk of developing AMD. A quarterly check-up by an ophthalmologist is also recommended in order to be able to diagnose the occurrence of AMD as early as possible.
Web links
Individual evidence
- ^ FC Donders: Contributions to the pathological anatomy of the eye. In: Archives for Ophthalmology. 1855; 1 (2), pp. 106-118.
- ↑ H. Müller: Investigations on the glass membranes of the eye, in particular the glass lamella of the choroid and their senile changes. In: Arch Ophthalmol. 1856; 2 (2), pp. 1-64.
- ^ WR Green: Histopathology of age-related macular degeneration. In: Mol Vis. 1999; 5, p. 27.
- ^ R. Klein, M. Davis, Y. Magli, P. Segal, B. Klein, L. Hubbard: The Wisconsin age-related maculopathy grading system. In: Ophthalmology. 1991; 98, pp. 1128-1134.
- ↑ M. Rudolf, ME Clark, MF Chimento, CM Li, NE Medeiros, CA Curcio: Prevalence and Morphology of Druse Types in the Macula and Periphery of Eyes with Age-Related Maculopathy. In: IOVS. 2008; 49, pp. 1200-1209.
- ↑ AA Khanifar, AF Koreishi, YES Izatt, CA Toth: Druze ultrastructure imaging with spectral domain optical coherence tomography in age-related macular degeneration. In: Ophthalmology. 2008; 115, pp. 1883-1890.
- ^ R. Klein, B. Klein, K. Linton: Prevalence of age-related maculopathy. In: Ophthalmology. 1992; 99, pp. 933-943.
- ↑ a b c G. S. Hageman et al: An Integrated Hypothesis that considers Drusen as Biomarkers of Immune-Mediated Processes at the RPE-Bruch's Membrane Interface in Aging and Age-Related Macular Degeneration. In: Prog Ret Eye Res. 2001; 20, pp. 705-732.
- ↑ C. Curcio, C. Millican, T. Bailey, H. Kruth: Accumulation of cholesterol with age in human Bruch's membrane. In: IOVS. 2001; 42, pp. 265-274.
- ^ AC Bird et al: An International Classification and Grading System for Age-related Maculopathy and Age-related Macular Degeneration. In: Surv Ophthalmol. 1995; 39, pp. 367-374.
- ^ The Age-Related Eye Disease Study Group. The Age-Related Eye Disease Study (AREDS): design implications. AREDS report no. 1. In: Control Clin Trials 1999; 20, pp. 573-600.
- ^ The Age-Related Eye Disease Study Group. Risk factors associated with age-related macular degeneration. A case-control study in the age-related eye disease study: Age-Related Eye Disease Study Report Number 3. In: Ophthalmol. 2000; 107, pp. 2224-2232.
- ^ NM Bressler et al.: Five-year incidence and disappearance of drusen and retinal pigment epithelial abnormalities: Waterman Study. In: Arch Ophthalmol. 1995; 113, pp. 301-308.
- ↑ FG Holz et al .: Bilateral macular drusen in age-related macular degeneration: prognosis and risk factors. In: Ophthalmol. 1994; 101, pp. 1522-1528.
- ↑ G. Fishman, D. Apple, M. Goldberg: Retinal and pigment epithelial alterations over choroidal malignant melanomas. In: Ann Ophthalmol. 1975; 7, pp. 487-489.
- ↑ Y. D'Souza include: Ten year review of drusen-like lesions in mesangiocapillary glomerulonephritis F ii. In: IOVS. 2000; 41, p. 164.
- ↑ K. Evans et al .: Assessment of the phenotypic range seen in Doyne honeycomb retinal dystrophy. In: Arch Ophthalmol. 1997; 115, pp. 904-910.
- ↑ P. Polkinghorne et al .: Sorsby's fundus dystrophy. A clinical study. In: Ophthalmol. 1989; 96, pp. 1763-1768.
- ^ The Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. In: Arch Ophthalmol. 2001; 119, pp. 1417-1436.