Electrochemical scanning microscopy
Electrochemical scanning microscopy ( SECM ) ( English: scanning electrochemical microscopy ) is a technique from the larger class of scanning probe microscopy that is used to investigate the behavior of phase boundaries liquid / solid, liquid / gas and solid / solid. The beginnings of this method go back to Allen J. Bard , an electrochemist from the University of Texas. Since the technology was also supported theoretically, it has been widely used in chemistry, biology and materials science. High-resolution electrochemical signals can be recorded by using an ultramicroelectrode (UME) to measure the current as a function of the position with the electrode tip at the relevant location on the substrate. The interpretation of the SECM signal is based on the concept of diffusion-limited electric current. Two-dimensional raster scan information can be used to measure images of surface reactivity and chemical kinetics . A complementary method for surface characterization is surface plasmon resonance spectroscopy , electrochemical scanning tunneling microscopy (ESTM), atomic force microscopy , also atomic force microscopy or atomic force microscopy (abbreviation (AFM)), when examining various surface phenomena. In addition to gaining information about the surface structure, SECM is often used to test the surface reactivity of solids, electrochemically active catalysts, enzymes and other biophysical systems. SECM and variations of the technique have been used in microfabrication, surface finishing and microstructuring.
history
The need for ultramicroelectrodes ( UME ) around 1980 went hand in hand with the development of highly sensitive electroanalytical technology such as SECM. The use of tips made it possible to study rapid electrochemical reactions. The first SECM experiment was carried out in 1986 by Engstrom, who obtained direct observation of reaction profiles and short-lived intermediates. Simultaneous experiments by Alan J. Bard with the electrochemical scanning tunneling microscope ( ESTM ) showed a current over long distances "electrode tip to sample" which could not be explained by the tunnel effect. This phenomenon was based on the Faraday current , which enabled a careful analysis in electrochemical microscopy. The theoretical basis was laid by Bard in 1989 when the term electrochemical scanning microscopy was first coined. In addition to the data collection mode in use at the time, Bard expanded the widespread use of the SECM by adding various settings. When the theoretical background was explained, the number of annual publications grew from 10 to 80 in 1999, when the first SECM was commercially available. The popularity of the SECM is growing due to advances in theory and technology, which expand the experimental possibilities to a more diverse substance selection and to a finer accuracy.
Application principle
The electrical potential is generated by the UME tip in a saturated solution, e.g. B. contains the redox couple (Fe 2+ / Fe 3+ ). If the applied voltage is negative enough, (Fe 3+ ) at the electrode tip is reduced to (Fe 2+ ), creating a diffusion-controlled current. The measured current is based on the ion current in the solution to the electrode and is described mathematically by the equation:
where i T, ∞ is the diffusion-controlled current and, n is the number of electrons that reach the electrode tip (O + n e - → R), F is Faraday's constant , C is the concentration of the oxidized species in solution, D is is the diffusion coefficient and a is the radius of the UME electrode tip. In order to examine the corresponding surface, the tip is moved towards the surface and thus generates the current.
The two modes of operation are the feedback mode and the collecting mode.
Feedback mode
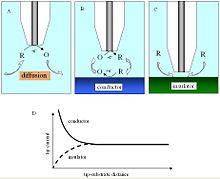
In a saturated solution, the oxidized species is reduced at the electrode tip, generating a current that is limited by the half-cell diffusion. As the electrode tip approaches a conductive substance in solution, the reduced species formed at the tip is oxidized on the conductive surface by generating a current at the tip, creating a positive feedback loop. The opposite effect is observed when examining insulating surfaces as the oxidized species cannot be regenerated and diffusion to the electrode is prevented (as a result of physical inhibition) as the tip approaches the substrate, creating a negative feedback loop and decreased the current at the top. An additional parameter to consider when examining insulating surfaces is the parameter r g , as it is used to physically inhibit diffusion.
The change in the current at the electrode tip can be plotted as an approximate curve as a function of the distance d . Due to the concentration dependence of the SECM measurements, one can also investigate electron transfer kinetics.
Collect mode
Another mode of operation is used in tip creation / substrate collection (TG / SC). In this mode, the tip is held at a voltage high enough for the electrode to react so that a product can be created that can react with the substrate or so that the electrode product can be picked up by the substrate. The reverse method is substrate creation / tip collection (SG / TC), where the substrate creates the species that is measured (or collected) at the tip.
Two streams are produced: the peak current i T , and the substrate current i S . Once the substrate is much larger than the tip, the effectiveness of collecting i S / i T is 1 if there is no reaction during the transition of the tip-generated species to the substrate. As the tip-substrate distance d decreases, the collection efficiency approaches 1.
SECM imaging
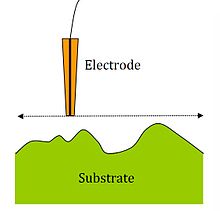
Changing the current as a function of the distance between the electrode tip and the solution surface allows the mapping of insulating and conductive surfaces. Information about the nature and reactivity is obtained by moving the tip over the surface and thus generating the current. The most common scanning method is constant height, where the tip height is unchanged while the surface is scanned in the XY plane. An alternative is to measure at constant current, where one tries to generate a constant current as the substrate / tip distance changes and to record the changes in d .
The resolution depends on the tip radius, the distance from the substrate to the tip, the precision of the electronic instruments and other things.
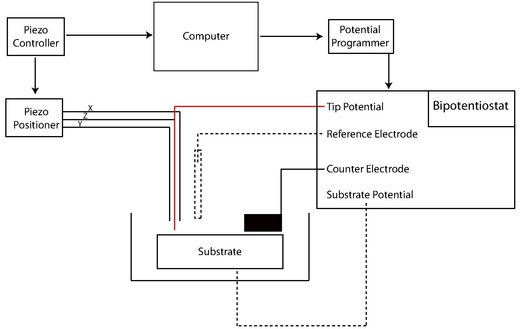
Instrumental structure
The early SECMs were constructed by individual working groups with the generally used devices: potentiostat and bipotentiostat, voltage transmitter, current amplifier, piezoelectric positioner and piezoelectric measuring device, computer and the UME (electrode). Some SEMC experiments are highly specific and home-made SEMCs remain common. The development of new techniques in the direction of nanofabrication of the electrodes has long been the focus of the relevant literature because of the decisive advantages including the high concentrations and the low-threshold absorption of reactants in kinetic experiments. In addition to the small tip size required for high resolution, SECM research tends towards smaller and faster phenomena. The following methods give a brief overview of the manufacturing techniques in the rapidly developing field.
Prepare the electrodes
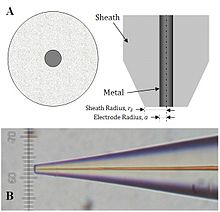
The typical production of a microscale electrode is done by heating a micro-carbon fiber cable in a glass capillary under vacuum. This tip can be tied to a slightly larger copper electrode with the help of silver epoxy and then polished to give a sharp tip. The electrodes are nano-colored by etching the metal with NaCN / NaOH. The etched metal cable is then coated with wax, polished and sheathed with paraffin, glass or polymethylstyrene or polyimide , electropolymerized with phenol and electrophoretically colored / lacquered. Nanotips produced in this way are conical. Disc-shaped tips are obtained by pulling glass-coated electrodes like a micropipette. Nanoscale electrodes allow high-resolution experiments for biological applications on a microscale or single-molecule experiments . Experiments in which the tip is immersed in a microstructure (e.g. polymer films with a fixed redox center) to test kinetic parameters and concentrations also require nanoscale electrodes. However, microelectrodes remain ideal for quantitative kinetics experiments because of the larger surface area.
Different types of electrodes have been developed in addition to the different size variations. SECM-AFM examinations can be used as a sensor or an electrode, due to the ground, etched metal cable with the sheathing and the electrophoretically produced paint. In this system, the smooth cable is used as a flexible cantilever to measure the counterforce of the sample (AFM) while the electrode cable measures the current. In addition, the SECM functionality can be integrated into a standard AFM by finishing the surface with a conductive material or treating an insulated tip with a focusing ion beam. Only recently was it shown using electron current lithography that reproducible SECM-AFM examinations can be carried out with silicon dioxide wafers .
Images of the chemical environment independent of local topographies are desirable for investigating larger or uneven surfaces. Soft style studies have recently been developed by creating a microfabricated trace on a sheet of polyethylene terephthalate with a conductive coal mine. Lamination with a polymer film created a v-shaped pin that was cut off to expose the carbon tip. The flexible design allows constant contact between the tip and the substrate to be examined. If the apparatus continues with the tip across the sample, the topographical differences at a substantially constant peak-to-substrate distance, d .
Micro-ITIES examinations are another special examination method that takes advantage of the phase boundary between two immiscible electrolyte solutions. The tip forms an extended pipette containing a metallic counting electrode and is used to measure electron and ion transfer processes during the mixing process while a counting reference electrode is immersed in the immiscible liquid.
Often a phase boundary liquid / liquid and gaseous / liquid is measured with an SECM underwater electrode. The growing complexity of electrodes decreases with size and requires high-resolution technology. Atomic force microscopy ( scanning electron microscopy , SEM), cyclic voltammetry and SECM approximation curves are used to describe the dimensions and geometries of the experiments.
Potentiostat
The potentiostat measures the voltage of the three-electrode system for voltammetric experiments . The UME is the working electrode for applying a controlled voltage to the substrate. The auxiliary electrode (or counting electrode) regulates the current that is generated at the working electrode, often by redox reduction with the solvent or an electrolyte additive. The voltage is measured relative to a precisely defined reduction potential of the reference electrode, through which no current itself flows.
Positioner and converter
The SECM uses the same positioning components that other characterization techniques use. Precise positioning between tip and sample is a critical factor that is inversely proportional to tip size. The positioning of the sample relative to a certain point on the material surface in the x, y and z directions is typically adjusted by a motor for coarse positioning, linked to a piezoelectric motor for fine adjustment. More precisely, the systems have a motor in the inch range and an additional piezo motor in the z-direction. Stepper motors with an XYZ piezo block or closed loop control are also used.
Applications
SEMC was used to measure the surface properties and surface reactivity of a solid, to research the kinetics of the dissociation of ion crystals in aqueous solution, to visualize electrocatalytic processes, to illustrate enzymatic reactions and to investigate the dynamics of transport across a membrane or through other biophysical systems . Early experiments focused on the solid / liquid interface and the characterization of solvent-based electrochemical systems at higher resolution and sensitivity than conventional electrochemical systems typically offer. Recently, a SEMC technique was developed to investigate the chemical dynamics at the liquid / liquid and liquid / gaseous phase boundaries.
Solid / liquid phase boundary
Microstructure
SEMC and variants of the technology have found application in microfabrication, surface finishing and microstructuring. A variety of surface reactions was researched in this context - including metal decay - as well as the etching and structuring of surfaces with enzymes. The scanning probe lithography (SPL) of surfaces can also be carried out with SECM technology. Because of the limited possibilities in microfabrication of the UMEs, the resolution is reduced and requires larger sizes compared to SPL techniques. An earlier example showed the pattern of dodecyl thiolate, a self-assembling monolayer (SAM), by moving the UME in a two-dimensional field near the surface while applying a voltage on the order of the oxidation or reduction potential so that the chemical species is local is desorbed. Microscale patterns are effectively applied to the sample (SAM). A particular advantage of SECM compared to earlier SPL techniques for surface structuring is that electrochemical information is obtained at the same time while working lithographically. Other studies have shown the utility of SECM in applying local gold layers as templates to biomolecules and fluorescent dyes . Such techniques have been suggested as a potential technique for the production of nanoscale assemblies , as they make it possible to provide the investigated systems with small gold platelets.
Variations of the SECM were used because the micropipette-shaped tip can be used to apply spatially resolved microcrystals to a glass. Here, submicron glass capillaries are used, replacing the standard UME, and creating femtoliter- sized droplets that are dropped from a capillary onto a conductive surface that is used as a working electrode. Upon contact with a positively charged surface, the small droplets of the salt solution become supersaturation and crystallize with a well-defined microscale geometry.
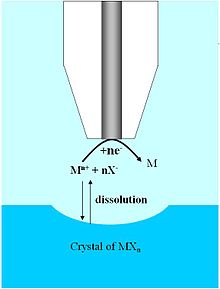
Ion dissociation
The dissociation of ion crystals in aqueous solution is important for the characterization of the host in naturally occurring and synthetic host-guest systems. The high spatial resolution and the three-dimensional mobility that is given with the UME make it possible to measure the dissociation kinetics of special ion crystals, whereby with earlier characterization techniques only a measurement of the total mass was possible, which was then averaged. Because of the high mass transfer rates measured with UME and SECM, it is possible to measure very fast reaction kinetics. In addition, the UME allows measurement over a large dynamic space and thus enables the investigation of ionic solids with very large differences in solubility. The first examples of the application of the SECM were carried out with saturated copper sulfate solution. By positioning a UME and SECM configuration, approximately at the distance of the electrode radius from the crystal face of the copper sulfate, it was possible to destroy the dissociation equilibrium through local reduction at the electrode surface. The crystal surface, which is separated into copper and sulfate ions, shows a visible groove and a chrono-amperometric signal could be recorded as a function of the distance between crystal and electrode. Assuming first and second order reactions, the reaction constant results from the measured data. Similar studies have been performed on crystal systems with no supporting electrolytes.
Electrocatalytic Investigation
The search for new catalytic materials to replace the precious metals used for fuel cells requires precise knowledge of the redox reactions on metal surfaces. Even more important is the need to study a large number of new electrocatalytically active substances. Some groups working on electrocatalysis have shown that SECM is a rapid screening technique that provides locally quantitative electrochemical information about catalyst materials and properties.
A variety of concepts have been proposed for an optimal selection of new metallic electrocatalysts. A functional, non-SECM experiment made it possible for a large number of optically active catalysts to be investigated, using a technique that detects proton production with proton-sensitive dyes. While this technique is useful, it lacks exact quantitative electrochemical information that can only be obtained outside of the experimental setup. Bard et al. have now developed a method that can measure electrocatalytic activities at large volumes with the SECM. With approximation methods, direct quantitative electrochemical information from multicomponent systems can be obtained on a rapid screening platform. These high throughput screening processes enable a reliable, effective and cost-effective selection of electrocatalytic materials to replace platinum and other precious metals.
Biological analysis
The ability to study non-conductive surfaces makes SECM a useful method to analyze membranes, redox-active enzymes, and other biophysical systems. Changes in intracellular redox activity are related to oxidative stress and cancer. Redox processes in living cells can be examined with SECM, a non-invasive method to record intracellular charge transfer. During such measurements, the cell in question is fixed on a surface, wetted with a solution of the oxidized form of the redox couple, and the feedback mode is set. A voltage is applied to the tip which reduces the oxidized species while the current i T is generated. When the top product gets into the cell, it is re-oxidized there by processes in the cell and excreted again. The peak current changes depending on the concentration rate at which the peak product is regenerated in the cell. Liu et al. applied this method and showed that the redox states of human breast cells have different consistencies (mobile, immobile and metastatic). SECM can not only study immobilized cells, but also the kinetics of immobilized redox-active enzymes.
The transport of ions such as K + and Na + through membranes and other biological phase boundaries is vital for many cells; SECM was used to study the transport of redox-active species across cell membranes. In feedback mode, the transport of molecules through a membrane is made possible by collecting the transferred species at the electrode tip and by forming a concentration gradient that enables diffusion processes. The changes in current can be measured as a function of the transport rate of molecules.
Liquid / liquid phase boundary
Electrocatalysis
The phase boundary between two immiscible electrolyte solutions can be investigated with the help of SECM with micro-ITIES. The investigation takes place in a phase and is moved to the phase boundary while a potential is applied. Oxidation and reduction change the concentration of the substrate, which results in diffusion from one phase to the other. When the tip-phase boundary is close, a substrate or ions can be examined with regard to their diffusion rate between the aqueous and organic phases. Electron transfer has also been researched with ITIES. In these experiments, redox couples are dissolved in two different phases and the current generated is recorded with the ITIES. This is exactly the fundamental principle in the investigation of transport phenomena in membranes.
Phase boundary liquid / gaseous
The greatest gain in this area so far has been the quantitative analysis of molecular movement along a single-layer film in order to gain knowledge about chemical transport properties in cell membranes and chemical diffusion into the solution surrounding them.
Although much work has been done in the area of evaporation (evaporation) through simple membranes, the invention of SEMC scientists provided the opportunity to study the permeability of simple membranes to small soluble molecules across the phase boundary. By precisely positioning an underwater electrode next to an organic single membrane that separates an air-water phase boundary, scientists were able to investigate the diffusion equilibrium of oxygen by locally reducing the amount of oxygen in the aqueous phase, whereby the diffusion through the membrane was recorded. Diffusion dynamics of a system can be illustrated by measuring an electrical current in response to the UME with high spatial and temporal resolution. SECM is a very suitable option for kinetic studies, since the current can be absorbed with high sensitivity (depending on SECM and UME) due to the rapid mass transfer. The mobility of the UME in all three spatial directions offers an examination method with which the points for the permeability can be measured. A similar approach is used for studies of the liquid / liquid and solid / liquid phase boundary.
Individual evidence
- ↑ a b c Unwin, Patrick, Barker, Gonsalves, Macpherson, Slevin: Scanning electrochemical microscopy: beyond the solid / liquid interface . In: Elsevier: Analytica chimica acta . 385, 1999, pp. 223-240.
- ↑ a b Zhang, Jie, Barker, Unwin: Measurement of the forward and back rate constants for electron transfer at the interface between two immiscible electrolyte solutions using scanning electrochemical microscopy (SECM): Theory and experiment . In: Electrochemistry Communications . 3, 2001, pp. 372-378. doi : 10.1016 / s1388-2481 (01) 00173-4 .
- ^ Mirkin, Michael, Sun: Kinetics of electron transfer reactions at nanoelectrodes . In: Analytical Chemistry . 78, 2006, pp. 6526-6534. doi : 10.1021 / ac060924q .
- ^ A b c Mirkin, Michael, Peng Sun, Francois O. Laforge: Scanning electrochemical microscopy in the 21st century . In: Physical Chemistry Chemical Physics . 9, 2007, pp. 802-823. bibcode : 2007PCCP .... 9..802S . doi : 10.1039 / b612259k .
- ^ Wittstock, Gunther: Imaging Localized Reactivities of Surfaces by Scanning Electrochemical Microscopy . In: Topics in Applied Physics . 85, 2003, pp. 335-366. doi : 10.1007 / 3-540-44817-9_11 .
- ↑ a b c d Bard, Allen, Fan, Fu Ren F., Kwak, Juhyoun., Lev, Ovadia .: Scanning electrochemical microscopy. Introduction and principles . In: Analytical Chemistry . 61, No. 2, 1989, ISSN 0003-2700 , pp. 132-138. doi : 10.1021 / ac00177a011 .
- ↑ a b c d Bard, Allen: Scanning Electrochemical Microscopy . Marcel Dekker, New York 2001, ISBN 0-8247-0471-1 .
- ↑ Szunerits, Sabine, Knorr, Nikolaus, Calemczuk, Roberto, Livache, Thierry: New Approach to Writing and Simultaneous Reading of Micropatterns: Combining Surface Plasmon Resonance Imaging with Scanning Electrochemical Microscopy (SECM) . In: Langmuir . 20, No. 21, 2004, ISSN 0743-7463 , pp. 9236-9241. doi : 10.1021 / la0492557 .
- ^ Wittstock, Gunther, Thomas H. Treutler: Combination of an electrochemical tunneling microscope (ECSTM) and a scanning electrochemical microscope (SECM): application for tip-induced modification of self-assembled monolayers . In: Electrochimica Acta . 48, 2003, pp. 2923-2932. doi : 10.1016 / s0013-4686 (03) 00357-8 .
- ↑ Mizaikoff, Bertagnolli, Lugstein, Kueng, Kranz: Mapping of enzyme activity by detection of enzymatic products during AFM imaging with integrated SECM AFM probes . In: Ultramicroscopy . 100, 2004, pp. 127-134. doi : 10.1016 / j.ultramic.2003.10.004 .
- ^ Wittstock, Gunther, Burchardt, Malte, Pust, Sascha, Shen, Yan, Zhao, Chuan: Scanning Electrochemical Microscopy for Direct Imaging of Reaction Rates . In: Angewandte Chemie International Edition . 46, No. 10, 2007, ISSN 1433-7851 , pp. 1584-1617. doi : 10.1002 / anie.2006027500 .
- ↑ a b c Gorman, Christopher, Stephan, Kramer, Ryan R., Fuierer: Scanning Probe Lithography Using Self-Assembled Monolayers . In: Chemical Reviews . 103, 2003, pp. 4367-4418. doi : 10.1021 / cr020704m .
- ↑ a b Engstrom, RC, M. Weber, DJ Wunder, R. Burgess, S. Winguist: Measurements within the diffusion layer using a microelectrode probe . In: Anal. Chem. . 58, No. 4, April 1986, pp. 844-848. doi : 10.1021 / ac00295a044 .
- ↑ Bard, Allen, Hsue-Yang Liu, Fu-Ren F. Fan, Charles W. Lin: Scanning Electrochemical and Tunneling Ultramicroelectrode Microscope for High-Resolution Examination of Electrode Surfaces in Solution . In: J. Am. Chem. Soc. . 108, 1986, pp. 3838-3839. doi : 10.1021 / ja00273a054 .
- ^ A b Mirkin, Michael, Peng Sun, Francois Laforge: Scanning electrochemical microscopy in the 21st century . In: Physical Chemistry Chemical Physics . 9, November 30, 2006, pp. 802-23. bibcode : 2007PCCP .... 9..802S . doi : 10.1039 / b612259k . Retrieved October 5, 2011.
- ↑ Michael V. Mirkin, Wojciech Nogala, Jeyavel Velmurugan, Yixian Wang: Scanning electrochemical microscopy in the 21st century. Update 1: five years after . In: Physical Chemistry Chemical Physics . tape 13 , no. 48 , November 29, 2011, pp. 21196-21212 , doi : 10.1039 / C1CP22376C .
- ^ Bard, Allen J., David O. Wipf: Effect of Heterogeneous Electron-Transfer Rate at the Substrate on the Tip Feedback Current . In: J. Electrochem. Soc. . 138, No. 2, 1991, pp. 469-474.
- ↑ Schuhmann, Wolfgang, Bernardo Ballesteros Katemann, Albert Schulte: Constant-Distance Mode Scanning Electrochemical Microscopy. Part II: High-Resolution SECM Imaging Employing Pt Nanoelectrodes as Miniaturized Scanning Probes . In: Electroanalysis . 16, No. 1-2, 2004, pp. 60-65. doi : 10.1002 / elan.200302918 .
- ↑ Unwin, Patrick, Martin A Edwards, Sophie Martin, Anna L Whitworth, Julie V Macpherson: Scanning electrochemical microscopy: principles and applications to biophysical systems . In: Physiological Measurement . 27, 2006, pp. R63-R108. bibcode : 2006PhyM ... 27R..63E . doi : 10.1088 / 0967-3334 / 27/12 / R01 . Retrieved October 5, 2011.
- ^ P. Sun, Z. Zhang, J. Guo, Y. Shao: Fabrication of nanometer-sized electrodes and tips for scanning electrochemical microscopy . In: Analytical Chemistry . tape 73 , no. 21 , November 1, 2001, pp. 5346-5351 , PMID 11721940 .
- ↑ Christopher J Slevin, Nicola J Gray, Julie V Macpherson, Mark A Webb, Patrick R Unwin: Fabrication and characterization of nanometer-sized platinum electrodes for voltammetric analysis and imaging . In: Electrochemistry Communications . tape 1 , no. 7 , July 1, 1999, p. 282-288 , doi : 10.1016 / S1388-2481 (99) 00059-4 .
- ↑ Amemiya S, Bard AJ, Fan FR, Mirkin MV, Unwin PR. Annu Rev Anal Chem (Palo Alto Calif). 2008; 1: 95-131
- ↑ Phillip S. Dobson, John MR Weaver, Mark N. Holder, Patrick R. Unwin, Julie V. Macpherson: Characterization of Batch-Microfabricated Scanning Electrochemical-Atomic Force Microscopy Probes . In: Analytical Chemistry . tape 77 , no. 2 , January 1, 2005, p. 424-434 , doi : 10.1021 / ac048930e .
- ↑ Fernando Cortés-Salazar, Markus Träuble, Fei Li, Jean-Marc Busnel, Anne-Laure Gassner, Mohamad Hojeij, Gunther Wittstock, Hubert H Girault. "Soft Stylus Probes for Scanning Electrochemical Microscopy" Analytical Chemistry Vol. 18, Issue 16. Date: 08/15/2009 Start Page: 6889.
- ↑ Unwin, Patrick, Jie Zhang, Christopher J. Slevin, Colin Morton, Peter Scott, David J. Walton: New Approach for Measuring Lateral Diffusion in Langmuir Monolayers by Scanning Electrochemical Microscopy (SECM): Theory and Application . In: Journal of Physical Chemistry B . 105, 2001, pp. 11120-11130. doi : 10.1021 / jp004592j .
- ↑ Mandler, Daniel, Tomokazue Matsue, Iva Turyan: Patterning and Characterization of Surfaces with Organic and Biological Molecules by the Scanning Electrochemical Microscope . In: Analytical Chemistry . 72, 2000, pp. 3431-3435. doi : 10.1021 / ac000046a .
- ↑ Zhong-Qun, Dezhi Yang, Lianhuan Han, Yang Yang, Liu-Bin Zhao, Cheng Zong, Yi-Fan Huang, Dongping Zhan: Solid-State Redox Solutions: Microfabrication and Electrochemistry . In: Angewandte Chemie . 50, 2011, pp. 8679-8682. doi : 10.1002 / anie.201103386 .
- ↑ Unwin, Patrick, Julie Macpherson: Oscillatory Dissolution of an Ionic Single Crystal Surface Observed with the Scanning Electrochemical Microscope . In: The Journal of Physical Chemistry . 98, 1994, pp. 11764-11770. doi : 10.1021 / j100096a022 .
- ↑ Unwin, Patrick, Julie Macpherson: A Novel Approach to the Study of Dissolution Kinetics Using the Scanning Electrochemical Microscope: Theory and Application to Copper Sulfate Pentahydrate Dissolution in Aqueous Sulfuric Acid Solutions . In: The Journal of Physical Chemistry . 98, 1993, pp. 1704-1713. doi : 10.1021 / j100057a026 .
- ↑ Unwin, Patrick, Julie Macpherson: Scanning Electrochemical Microscope-Induced Dissolution: Theory and Experiment for Silver Chloride Dissolution Kinetics in Aqueous Solution without Supporting Electrolyte . In: The Journal of Physical Chemistry . 100, 1996, pp. 19475-19483. doi : 10.1021 / jp9614862 .
- ↑ a b Bard, Allen, Walsh, Fernandez: Thermodynamic Guidelines for the Design of Bimetallic Catalysts for Oxygen Electroreduction and Rapid Screening by Scanning Electrochemical Microscopy. M-Co (M: Pd, Ag, Au) . In: JACS . 127, 2005, pp. 357-365. doi : 10.1021 / ja0449729 .
- ^ Bard, Allen, Aguilar, Zoski: Scanning Electrochemical Microscopy. 46. Shielding Effects on Reversible and Quasi-Reversible Reactions . In: Analytical Chemistry . 75, 2003, pp. 2959-2966. doi : 10.1021 / ac034011x .
- ↑ Mallouk, Thomas, Erik Reddington, Anthony Sapienza, Bogdan Gurau, Rameshkrishnan Viswanathan S. Sarangapani, Eugene S. Smotkin: Combinatorial Electrochemistry: A Highly Parallel, Optical Screening Method for Discovery of Better Electrocatalysts . In: Science . 280, No. 5370, 1998, pp. 1735-1737. bibcode : 1998Sci ... 280.1735R . doi : 10.1126 / science.280.5370.1735 .
- ↑ Liu, Biao, Susan A. Rotenberg, Michael V. Mirkin: Scanning electrochemical microscopy of living cells: Different redox activities of nonmetastatic and metastatic human breast cells . In: PNAS . 97, No. 18, August 2000, pp. 9855-9860. bibcode : 2000PNAS ... 97.9855L . doi : 10.1073 / pnas.97.18.9855 .
- ↑ Pierce, David T., Patrick R. Unwin, Allen J. Bard: Scanning Electrochemical Microscopy: 17. Studies of Enzyme-Mediator Kinetics for Membrane and Surface Immobilized Glucose Oxidase . In: Anal. Chem. . 64, 1992, pp. 1795-1804. doi : 10.1021 / ac00041a011 .
- ↑ Mirkin, Michael, Yuanhua Shao: Probing Ion Transfer at the Liquid / Liquid Interface by Scanning Electrochemical Microscopy (SECM) . In: Journal of Physical Chemistry B . 102, October 30, 1998, pp. 9915-9921. doi : 10.1021 / jp9828282 . Retrieved October 6, 2011.
- ↑ LJ Thibodeaux: Environmental Chemo Dynamics: Movement of Chemicals in Air, Water and Soil 1996th
- ↑ Patrick Unwin, Christopher J. Slevin, Steve Ryley, David J. Walton: A New Approach for Measuring the Effect of a Monolayer on Molecular Transfer across an Air / Water Interface Using Scanning Electrochemical Microscopy . In: Langmuir . 14, No. 19, 1998, pp. 5331-5334. doi : 10.1021 / la980320k .