Electron shift
Under electron translation is generally understood as the motion of a single electron or a lone pair , in the course of a chemical reaction takes place. To illustrate this, the displacement is shown using curved arrows ( ). The movement of an electron or electron pair from an occupied to a free orbital is illustrated. This technique was developed by Robert Robinson in 1922 and has since been widely used in the representation of reaction mechanisms . In chemical reactions, the overlap and addition of molecular orbitals to form new molecular orbitals, as well as the movement of electrons within these orbitals, play an important role. With the help of the curved arrows, important features of these interactions and electron movements can be shown. It should be noted that this formalism is only a simplification and that the shifting of electrons or the electron density does not actually take place step by step.
Representation of electron shifts
In organic chemistry, two types of curved arrows are used to represent electron displacement, as a distinction is made between the displacement of an electron pair and the displacement of a single electron.
Electron pair shift
The curved arrow with a two-pointed arrowhead illustrates the movement of two electrons (an electron pair) from an occupied to a free orbital. So they start from an electron-rich center and extend to an electron-poor center. When representing reaction mechanisms, the arrow therefore assumes a lone pair of electrons on an atom or an electron pair bond. The arrowhead mostly points to the atom to which the new bond is formed.
One electron shift
The one-electron shift is represented by single-pointed arrows in the shape of a fish hook. Since the transfer of individual electrons leads to the formation of radicals, these arrows are also known as radical arrows.
Bond cleavage
The products of a chemical reaction are the result of a process in which bonds are broken and new bonds are formed. Bonds between two atoms can be seen as a set consisting of two electrons, i.e. an electron pair. A single bond is therefore composed of two electrons, a double bond of four electrons and a triple bond of six electrons.
In general, a distinction is made between three ways of bond cleavage : heterolytic bond cleavage , homolytic bond cleavage and rearrangements such as occur in pericyclic reactions .
Heterolytic bond cleavage
A heterolytic bond cleavage (heterolysis) is used when the two bonding electrons remain exclusively with one of the two atoms involved. This leads to the formation of ions , with the fragment that receives the binding electrons being negatively charged. The other fragment receives a positive charge.
Homolytic bond cleavage
With homolytic bond cleavage (homolysis), the electron density is distributed equally to both atoms involved after the cleavage. Each atom receives a binding electron. The products of homolysis are radicals that have an unpaired electron, which is indicated by a dot on the atomic symbol. Radicals can be atoms and molecules.
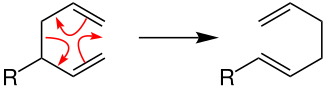
Pericyclic reactions
In the pericyclic reactions, the electrons move in a circle. When looking at the reaction mechanism, this is shown by the arrows tracing a ring and ending where they started. The bonds are broken and formed simultaneously so that there are no intermediate stages (and thus no positive or negative charges on intermediate stages). The example below of the Cope rearrangement (R = remainder) illustrates this reaction behavior in which neither free radicals nor ions are involved.
Another well-known example of a pericyclic reaction is the Diels-Alder reaction .
Further examples of electron shifts in chemistry
π-electron shift in dyes
If a molecule contains several conjugated double bonds, the π electrons can be shifted within the system, resulting in delocalization . The more conjugated a compound is, the smaller the energy difference between the highest electronically occupied ( HOMO ) and the lowest unoccupied molecular orbital (LUMO). This reduces the energetic distance between the ground state and the excited energy state of the molecule, the compound absorbs at higher wavelengths and thus appears colored ( bathochromic effect ). The azo dye 4-aminoazobenzene is therefore perceived, for example, in a yellow color.
Training of partial loads
If the binding partners of an atomic bond differ in their electronegativity , there is a shift in the electron density in favor of the more electronegative atom. The higher the electronegativity of an atom, the more it attracts the electron density in a bond. This leads to the formation of partial charges , which are marked with δ + and δ - above the element symbol.
literature
- Jonathan Clayden, Nick Greeves, Stuart Warren: Organic Chemistry . 2nd Edition. Springer Spectrum, Berlin / Heidelberg 2013, ISBN 978-3-642-34715-3 .
- Daniel E. Levy: Arrow pushing in organic chemistry: an easy approach to understanding reaction mechanism . Wiley, Hoboken, NJ 2008, ISBN 978-0-470-17110-3 .
Individual evidence
- ↑ a b c d Jonathan Clayden, Nick Greeves, Stuart Warren: Organic Chemistry . 2nd Edition. Springer Spectrum, Berlin / Heidelberg 2013, ISBN 978-3-642-34715-3 , p. 129-130 .
- ↑ William Ogilvy Kermack , Robert Robinson: bend left-An explanation of the property of induced polarity of atoms and of interpretation of the theory of partial valencies on to electronic basis . In: J. Chem. Soc., Trans. Volume 121 , 1922, pp. 427-440 , doi : 10.1039 / ct9222100427 .
- ↑ Paula Y. Bruice: Organic Chemistry: Study compact . 5th edition. Pearson Studium, Barcelona 2011, ISBN 978-3-86894-102-9 , pp. 135 .
- ↑ a b c Jonathan Clayden, Nick Greeves, Stuart Warren: Organic Chemistry . 2nd Edition. Springer Spectrum, Berlin / Heidelberg 2013, ISBN 978-3-642-34715-3 , p. 1064-1065 .
- ^ A b c Daniel E. Levy: Arrow pushing in organic chemistry: an easy approach to understanding reaction mechanisms . Wiley, Hoboken, NJ 2008, ISBN 978-0-470-17110-3 , pp. 1-5 .
- ^ A b Jonathan Clayden, Nick Greeves, Stuart Warren: Organic Chemistry . 2nd Edition. Springer Spectrum, Berlin / Heidelberg 2013, ISBN 978-3-642-34715-3 , p. 490; 961-963 .
- ↑ K. Peter, C. Vollhardt: Organic chemistry . 1st edition. VCH, Weinheim 1988, ISBN 3-527-26912-6 , pp. 618 .
- ↑ Jonathan Clayden, Nick Greeves, Stuart Warren: Organic Chemistry . 2nd Edition. Springer Spectrum, Berlin / Heidelberg 2013, ISBN 978-3-642-34715-3 , p. 165 .
- ↑ Entry on 4-aminoazobenzene. In: Römpp Online . Georg Thieme Verlag, accessed on November 24, 2018.
- ↑ Jonathan Clayden, Nick Greeves, Stuart Warren: Organic Chemistry . 2nd Edition. Springer Spectrum, Berlin / Heidelberg 2013, ISBN 978-3-642-34715-3 , p. 204 .