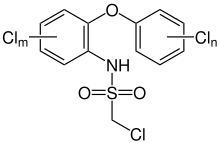
Chlorophenylide
Chlorphenylid or Eulan WA NEW (also Eulan U33 ) is a mixture of the two insecticidal forming agents PCSD and PCAD (often referred to as a collective term PCSD / PCAD due to the similar chemical structure). PCSD stands for the main component polychlor-2- (chloromethylsulfonamide) -diphenylether , also called chlorophenylide , and PCAD for the similar polychlor-2-aminodiphenylether . In the manufacturing process, PCAD is a technical impurity of the desired product PCSD.
History and use
The chemist Ernst Meckbach and the entomologist Erich Titschack researched the clothes moth intensively from 1919 onwards and developed the first moth repellent for wool, which was called Eulan , at the paint factory formerly Friedrich Bayer & Co. in Leverkusen . The further developed substance mixture Eulan® WA New , later Eulan U33 , was sold for decades as a textile and carpet protection agent against moths and beetles as well as for animal taxidermy . The chemical structure of the active ingredients it contains was kept secret. After the expiry of patent protection , similar products, some from Eastern Europe, are manufactured under the product names Mitex U33 , Mottine E liquid , Molantin P and Mitin . Bayer ceased production of the mixture in 1988. There are currently also newer fabrics that are manufactured under the name Eulan . These include halogenated diphenyl ethers similar to PCSD, such as Eulan SP , and halogenated derivatives of triphenylphosphine such as Eulan NK .
Eulanize
The verb eulanize is derived from the name Eulan for equipping textiles and furs with insect repellants . Eulan itself is a made-up word derived from the Greek word ευλή ( eulé ) for maggot or worm .
Eulanized rheumatism cat fur
Chemical properties and toxicology

Advertising poster by Walter Biedermann , around 1935
Like many other halogenated hydrocarbons, both substances contained in the Eulanen are chemically very stable and difficult to break down. Depending on the degree of chlorination, the boiling points are between 400 and 600 ° C. Tests that confirm cancer production are not yet known. However, the active ingredients can be found indoors, in the sea and in fish and their structure is similar to aromatic amines or polychlorinated dioxins . Information on the acute toxicity of the manufacturer's Eulan WA is based on an LD 50 value of 1000 mg / kg (rat); the active ingredient mixture has an LD 50 of 200 mg · kg −1 . Bayer also gave a NOEL value for rats of 3 mg per kg per day for the mixture . No data exist on the toxicity of the aromatic amines PCAD .
In a study by the Federal Environment Agency in 2003, PCSD and PCAD were found in 15% of 600 house dust samples . The arithmetic mean was 1.36 mg kg −1 . In an older study from 1996, significantly higher values were measured at 13.2 mg kg −1 .
See also
literature
- Why Eulan? An explanation for the textile trade , Frankfurt am Main 20, Grüneburgplatz, IG Farbenindustrie AG, [1934]
- Martina Homolka: Eulan - a biocide against keratin pests and its relevance in museum collections . Deutsches Historisches Museum Foundation, 2015 ISBN 978-3-86102-186-5 ( dhm.de PDF) - Part 2, 2015: ISBN 978-3-86102-186-5 ( dhm.de PDF)
- Hermann Stötterer: The modern fight against wool pests . In: Wool pests and their modern control . Pp. 55-78. Published by Farbenfabriken Bayer AG Leverkusen. Undated, after 1952. → Table of contents .
- G. Westöö, K. Norén, B. Egestad, L. Palmér, G. Blomkvist: Structures and ring closures of compounds from the mothproofing agent eulan wa neu. In: Analytica Chimica Acta . 155, 1983, pp. 293-298, doi: 10.1016 / S0003-2670 (00) 85608-1 .
Web links
- Eulan Films , German Historical Museum
Individual evidence
- ^ Adolf Herfs (1962): Professor Dr. Erich Titschack on his seventieth birthday. Pest Control Indicator 35 (6): 92-93. doi: 10.1007 / BF02332877
- ↑ Erich Titschack (1922): Contributions to a monograph of the clothes moth, Tineola biselliella Hum. Journal of Technical Biology 10: 1–168, panels I – IV.
- ↑ Hermann Stötter (1947): Modern Moth Remedies: History of the Development of the "Eulan". Angewandte Chemie A 59 (5/6): 145-150. doi: 10.1002 / anie.19470590504 .
- ^ PubChem : Connections with the name Eulan .
- ↑ Der Große Duden spelling , Mannheim, 15th expanded edition 1961.
- ↑ Arguk environmental laboratory: Eulan information sheet ( Memento from June 25, 2012 in the Internet Archive )
- ↑ a b Arguk environmental laboratory: Eulan WA NEW / Eulan U 33: Active ingredients and occurrence in house dust .
- ^ Federal Environment Agency: Children's Environment Survey 2003/06: House dust. February 2008 Umweltdaten.de (PDF; 527 kB).