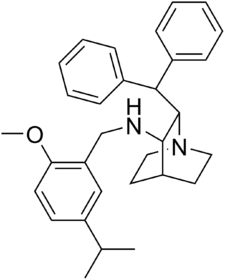
Ezlopitant
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||
| General | |||||||||||||
| Non-proprietary name | Ezlopitant | ||||||||||||
| other names |
|
||||||||||||
| Molecular formula | C 31 H 38 N 2 O | ||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| Drug information | |||||||||||||
| Drug class | |||||||||||||
| Mechanism of action | |||||||||||||
| properties | |||||||||||||
| Molar mass | 454.65 g · mol -1 | ||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Ezlopitant is a chemical compound that has been identified as a potential drug for the treatment of irritable bowel syndrome and for the treatment of nausea and vomiting after cytostatics as part of cancer chemotherapy . Its further clinical development by the pharmaceutical company Pfizer has been discontinued. Pharmacologically ezlopitant is a neurokinin - antagonist .
pharmacology
Mode of action (pharmacodynamics)
As an antagonist, ezlopitant inhibits the neurokinin NK 1 receptor . It displaces the body's own ligand substance P from its binding site on the receptor and thereby prevents the effects mediated by substance P. As an NK 1 receptor antagonist, ezlopitant has anti-inflammatory, analgesic, antidepressant and antiemetic potential. An effect that inhibits pain and vomiting has been confirmed in animal experiments. In addition, ezlopitant showed an appetite-suppressing effect in animal models. In clinical studies, ezlopitant has shown effectiveness in suppressing cytotoxic vomiting without improving the symptoms of nausea.
Pharmacokinetics
Ezlopitant is rapidly and almost completely absorbed into the systemic circulation after oral administration. It is subject to extensive, practically complete metabolism involving the cytochrome P450 isoenzymes CYP3A4 , CYP3A5 and CYP2D6 . The two main metabolites are produced by oxidation of the isopropyl side chain . The plasma half-life of ezlopitant is approximately 13 hours and that, including its metabolites, is over 100 hours. About 60 percent of the metabolites are excreted in the faeces and 40 percent in the urine.
literature
- Evangelista S: Eziopitant. Pfizer . In: Curr Opin Investig Drugs . 2, No. 10, October 2001, pp. 1441-1443. PMID 11890362 .
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ a b Evangelista S: Eziopitant. Pfizer . In: Curr Opin Investig Drugs . 2, No. 10, October 2001, pp. 1441-1443. PMID 11890362 .
- ↑ Tsuchiya M, Fujiwara Y, Kanai Y, et al. : Anti-emetic activity of the novel nonpeptide tachykinin NK1 receptor antagonist ezlopitant (CJ-11,974) against acute and delayed cisplatin-induced emesis in the ferret . In: Pharmacology . 66, No. 3, November 2002, pp. 144-152. PMID 12372904 .
- ↑ Tsuchiya M, Sakakibara A, Yamamoto M: A tachykinin NK1 receptor antagonist attenuates the 4 beta-phorbol-12-myristate-13-acetate-induced nociceptive behavior in the rat . In: Eur J Pharmacol . 507, No. 1-3, January 2005, pp. 29-34. doi : 10.1016 / j.ejphar.2004.11.028 . PMID 15659291 .
- ↑ Steensland P, Simms JA, Nielsen CK, Holgate J, Bito-Onon JJ, Bartlett SE: The neurokinin 1 receptor antagonist, ezlopitant, reduces appetitive responding for sucrose and ethanol . In: PLoS ONE . 5, No. 9, 2010. doi : 10.1371 / journal.pone.0012527 . PMID 20824145 . PMC 2931709 (free full text).
- ↑ a b Prakash C, O'Donnell J, Khojasteh-Bakht SC: Metabolism, pharmacokinetics, and excretion of a nonpeptidic substance P receptor antagonist, ezlopitant, in normal healthy male volunteers: characterization of polar metabolites by chemical derivatization with dansyl chloride . In: Drug Metab Dispos . 35, No. 7, July 2007, pp. 1071-1080. doi : 10.1124 / dmd.107.015362 . PMID 17431029 .
- ↑ Obach RS: Metabolism of ezlopitant, a nonpeptidic substance P receptor antagonist, in liver microsomes: enzyme kinetics, cytochrome P450 isoform identity, and in vitro-in vivo correlation . In: Drug Metab. Dispos. . 28, No. 9, September 2000, pp. 1069-76. PMID 10950851 .