Fabomotizol
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
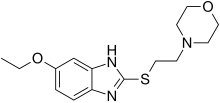
|
|||||||||||||
| General | |||||||||||||
| Non-proprietary name | Fabomotizol | ||||||||||||
| other names |
|
||||||||||||
| Molecular formula | C 15 H 21 N 3 O 2 S | ||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| Drug information | |||||||||||||
| ATC code | |||||||||||||
| properties | |||||||||||||
| Molar mass | 307.41 g mol −1 | ||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Fabomotizol is an anxiolytic drug that was introduced in Russia as Afobazol (tablets) in the early 2000s . In addition to anxiolysis, the active ingredient has neuroprotective properties.
In contrast to treatment with benzodiazepines , sedation and muscle relaxation do not occur during treatment with fabomotizole. The mechanism of action has so far been little researched. There is evidence of an effect on GABA receptors , melatonin receptor antagonism and sigma receptor agonism. Fabomotizol is a moderate reversible MAO-A inhibitor and also interacts with the serotonergic system. This appears to be potentially responsible for the anti-anxiety effects.
Clinical studies showed that Fabomotizol was well tolerated and is solidly effective in treating anxiety.
Individual evidence
- ↑ International Nonproprietary Names for Pharmaceutical Substances (INN) (= WHO Drug Information . No. 26 ). 1st edition. 2012, p. 63 ( Recommended International Nonproprietary Names: List 67 [PDF; accessed on March 21, 2015]).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ Fabomotizole hydrochloride , in: Martindale: The Complete Drug Reference, 38th Edition, Pharmaceutical Press, London 2014. p. 1073.
- ↑ GG Neznamov, SA Siuniakov, DV Chumakov, VK Bochkarev, SB Seredenin: Clinical study of the selective anxiolytic agent afobazol . In: Eksperimental'naia i klinicheskaia farmakologiia . 64, No. 2, 2001, pp. 15-9. PMID 11548440 .
- ↑ IV Silkina, TC Gan'shina, SB Seredin, RS Mirzoian: Gabaergic mechanism of cerebrovascular and neuroprotective effects of afobazole and picamilon . In: Eksperimental'naia i klinicheskaia farmakologiia . 68, No. 1, 2005, pp. 20-4. PMID 15786959 .
- ↑ SB Seredin, DS Melkumian, EA Val'dman, MA Iarkova, TC Seredina, MV Voronin, AS Lapitskaia: Effects of afobazole on the BDNF content in brain structures of inbred mice with different phenotypes of emotional stress reaction . In: Eksperimental'naia i klinicheskaia farmakologiia . 69, No. 3, 2006, pp. 3-6. PMID 16878488 .
- ↑ TA Antipova, DS Sapozhnikova, LIu Bakhtina, SB Seredenin: Selective anxiolytic afobazole increases the content of BDNF and NGF in cultured hippocampal HT-22 line neurons . In: Eksperimental'naia i klinicheskaia farmakologiia . 72, No. 1, 2009, pp. 12-4. PMID 19334503 .
- ↑ SB Seredenin, TA Antipova, MV Voronin, SY Kurchashova, AN Kuimov: Interaction of afobazole with sigma1-receptors . In: Bulletin of experimental biology and medicine . 148, No. 1, 2009, pp. 42-4. doi : 10.1007 / s10517-009-0624-x . PMID 19902093 .
- ↑ VE Medvedev, AP Trosnova, AV Dobrovol'skiĭ: Psychopharmacotherapy of anxiety disorders in patients with cardio-vascular diseases: the use of aphobazole . In: Zh Nevrol Psikhiatr Im SS Korsakova. . 107, No. 7, 2007, pp. 25-9. PMID 18379478 .