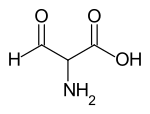
Formylglycine
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||
| Simplified structural formula without specifying the stereochemistry | |||||||||||||
| General | |||||||||||||
| Surname | Formylglycine | ||||||||||||
| other names |
|
||||||||||||
| Molecular formula | C 3 H 5 NO 3 | ||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 103.08 g mol −1 | ||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Formylglycine (FGly) or 3-oxoalanine or α-formylglycine is a non-proteinogenic amino acid . It occurs in the active center of some enzymes, such as sulfatases . Formylglycine is synthesized in prokaryotes and eukaryotes by the formylglycine-generating enzyme from a cysteine. The anaerobic sulfatase maturation enzyme AtsA or AtsB also exists in prokaryotes .
Formylglycine can also be used as a protein tag in genetic engineering .
α-Formylglycine should not be confused with N -formylglycine.
Stereoisomerism
Formylglycine is chiral . There are two enantiomers:
| Enantiomers of formylglycine | |
|---|---|
 |
 |
The 1: 1 mixture (racemate) of ( S ) -form and ( R ) -form is called ( RS ) -formylglycine.
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Maria Pia Cosma, Stefano Pepe, Ida Annunziata, Robert F. Newbold, Markus Grompe: The Multiple Sulfatase Deficiency Gene Encodes an Essential and Limiting Factor for the Activity of Sulfatases . In: Cell . tape 113 , no. 4 , May 16, 2003, ISSN 0092-8674 , p. 445-456 , doi : 10.1016 / S0092-8674 (03) 00348-9 ( cell.com [accessed January 23, 2020]).
- ↑ atsB - Anaerobic sulfatase-maturating enzyme - Klebsiella pneumoniae - atsB gene & protein. Retrieved January 23, 2020 .