Gangliosides
Gangliosides are mostly water-insoluble lipids from the group glycosphingolipids and glycosides , which occur in the outer half of the cell membrane of almost all vertebrates ; the membranes of nerve cells in particular are rich in gangliosides. The name is derived from “ganglion” ( “ nerve knot”) and the ending “-osid” (from “glycoside”).
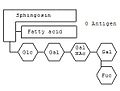
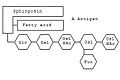
Gangliosides are anchored in the outer cell membrane through their fat-soluble content. Their structural framework is formed by the aminodialcohol sphingosine . Roughly speaking, gangliosides contain a complex oligosaccharide , a long-chain fatty acid and the sphingosine skeleton.
In contrast to the cerebrosides , they are more complex sphingolipids, which have branched sugar chains with up to seven sugar residues. These outwardly protruding sugar residues, especially negatively charged sialic acids , help determine the properties of the cell surface.
Occurrence
The highest concentrations of gangliosides are found in the nervous system , especially in the gray matter of the brain , where they make up 6% of all lipids. A large number of different gangliosides are accumulated in the nervous system, the composition of which changes in the course of development and when adapting to changing environmental conditions such as e.g. B. changing temperatures.
Medical benefit
In addition to their postulated importance in neuronal information transmission and storage, gangliosides are also important tumor markers. The occurrence of gangliosides in many cancers also indicates a role for these glycolipids in the progression of tumor diseases. However, it is still unclear which molecular mechanisms gangliosides work by.
blood type
The blood group can be determined using certain gangliosides . On sphingosine -Grundgerüst hangs a sphingolipid as with any fatty acid . The specialty of the gangliosides are the complex oligosaccharides, which are linked to the oxygen atom. Depending on which combination of monosaccharides is linked, determines the blood group. For example, the combination glucose (Glc) - galactose (Gal) - N-acetylgalactosamine (GalNAc) - galactose - fucose (Fuc) codes for blood group 0. If another N-acetylgalactosamine is attached to the chain , it codes for blood group A. ; instead another galactose is attached - for blood group B.
Degradation and pathology
The ganglioside breakdown takes place in the lysosomes . It takes place through highly specific glycosyl hydrolases, which sequentially split off the terminal sugar residues. The GM1 ganglioside is the master ganglioside; GM2 and GM3 ganglioside are formed as intermediate products during degradation. Disturbances in ganglioside breakdown can lead to serious diseases ( lysosomal storage diseases ), the sphingolipidoses . The accumulation of GM1 ganglioside in the central nervous system caused by the β-galactosidase deficiency explains the progressive cerebral symptoms in patients with GM1 gangliosidosis . Hexosaminidases A and B are responsible for the degradation of the GM2 ganglioside . Thus, the defect of the hexosaminidase A ends with the autosomal - recessive inherited Tay-Sachs disease , which is accompanied by an increase in the concentration of the ganglioside GM2, untreated before reaching the third year fatal. However, through amniocentesis and examination of the amniotic fluid for beta-N-acetylhexosaminidase activity, Tay-Sachs disease can already be diagnosed during the fetal development phase. A deficiency in α-galactosidase A causes globotriaosylceramide to accumulate , which causes Fabry disease.
literature
- Jeremy M. Berg, John L. Tymoczko, Lubert Stryer : Biochemistry. 6th edition. Elsevier - Spektrum Akademischer Verlag, Heidelberg 2007, ISBN 978-3-8274-1800-5 .
- Donald Voet, Judith G. Voet: Biochemistry. 3. Edition. John Wiley & Sons, New York NY et al. 2004, ISBN 0-471-19350-X .
- Bruce Alberts , Alexander Johnson, Peter Walter , Julian Lewis, Martin Raff , Keith Roberts: Molecular Biology of the Cell. 5th edition. Garland Science, New York, NY et al. 2008, ISBN 978-0-8153-4106-2 .
Individual evidence
- ↑ Sandro Sonnino, Laura Mauri, Vanna Chigorno, Alessandro Prinetti: Gangliosides as components of lipid membrane domains. In: Glycobiology. Vol. 17, No. 1, 2007, pp. 1R-13R, PMID 16982663 , doi : 10.1093 / glycob / cwl052 .
- ↑ Thomas Kolter, Richard L. Proia, Konrad Sandhoff : Combinatorial ganglioside biosynthesis. In: The Journal of Biological Chemistry . Vol. 277, No. 29, 2002, pp. 25859-25862, PMID 12011101 , doi : 10.1074 / jbc.R200001200 .
- ↑ Gerhild van Echten, Konrad Sandhoff: Ganglioside metabolism. Enzymology, Topology, and Regulation. In: The Journal of Biological Chemistry. Vol. 268, No. 8, 1993, pp. 5341-5344, PMID 8449895 , online (PDF; 535.12 kB) .
- ↑ Toshio Ariga, Michael P. McDonald, Robert K. Yu: Role of ganglioside metabolism in the pathogenesis of Alzheimer's disease - a review. In: The Journal of Lipid Research. Vol. 49, No. 6, 2008, pp. 1157-1175, PMID 18334715 , doi : 10.1194 / jlr.R800007-JLR200 .
- ↑ Purna Mukherjee, Anthony C. Faber, Laura M. Shelton, Rena C. Baek, Thomas C. Chiles, Thomas N. Seyfried: Ganglioside GM3 suppresses the proangiogenic effects of vascular endothelial growth factor and ganglioside GD1a. In: The Journal of Lipid Research. Vol. 49, No. 5, 2008, pp. 929-938, PMID 18287616 , doi : 10.1194 / jlr.M800002-JLR200 .
- ↑ Robert K. Yu, Erhard Bieberich, Tian Xia, Guichao Zeng: Regulation of ganglioside biosynthesis in the nervous system. In: The Journal of Lipid Research. Vol. 45, No. 5, 2004, pp. 783-793, PMID 15087476 , doi : 10.1194 / jlr.R300020-JLR200 .
- ↑ Entry on gangliosides. In: Römpp Online . Georg Thieme Verlag, accessed on November 4, 2013.
- ↑ Peter Nuhn : Naturstoffchemie. Microbial, vegetable and animal natural substances. 2nd, revised and expanded edition. S. Hirzel Wissenschaftliche Verlagsgesellschaft, Stuttgart 1990, ISBN 3-7776-0473-9 , pp. 324–326.