Fat hardening
Fat hardening is a process in which fatty oils are solidified. The double bonds of the unsaturated fatty acid residues are thereby saturated with hydrogen - in the presence of suitable catalysts (nickel) - by hydrogenation . From the (poly) unsaturated fatty acid glycerol esters (e.g. in vegetable oils ), glycerol esters of saturated fatty acids are formed. In the process, oils are converted into solid fats.
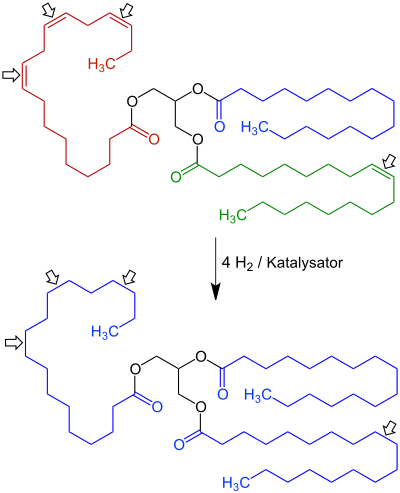
From relatively inexpensive and readily available vegetable oils, products are obtained that have better technical properties than natural (solid) fats such as butter or lard . In addition to solidification (higher melting point ), better storage life and an increased smoke point are achieved.
Typical foods that contain hydrogenated fats are margarine , fats for deep-frying , many types of biscuits and packaged cakes, ready-made meals - e.g. B. breaded fish and spreads such as peanut butter . Hydrogenated fats are also used to make soap .
Fat hardening was invented by Wilhelm Normann in 1901 .
Health risks
If the hydrogenation is incomplete, glycerides of the so-called trans fatty acids can be formed as a by-product, which are considered to be a contributory cause of cardiovascular diseases . Efforts are being made to minimize the amount of undesired trans fatty acid residues produced by a clever choice of catalysts .
literature
- Written question P-3109/03 by Phillip Whitehead (PSE) to the Commission (17 October 2003) Subject: Hydrogenated fats . In: Official Journal of the European Union . C 84E, April 3, 2004, p. 501.
Individual evidence
- ↑ Otto-Albrecht Neumüller (Ed.): Römpps Chemie-Lexikon. Volume 2: Cm-G. 8th revised and expanded edition. Franckh'sche Verlagshandlung, Stuttgart 1981, ISBN 3-440-04512-9 , pp. 1265-1270.
- ↑ To Philippaerts, Pierre A. Jacobs, Bert F. Sels: Does the hydrogenation of vegetable oils still have a future ?, Angew. Chem. 125 (2013) 5328-5334, doi : 10.1002 / anie.201209731 .