Gross fragmentation
|
Model representation of the coarse fragmentation using a triangle. |
The coarse fragmentation (also heterolytic fragmentation) is led under the term name reaction of organic chemistry . Cyril A. Grob (1917–2003) describes the Grob fragmentation more precisely, however, in general chemical reactions in which a compound is broken down into three fragments (Latin: fragment , “fraction” or “fragment”). According to Grob, this procedure can be used, for example, to clarify structures.
In 1967 Grob emphasized that, from a chemical point of view, the term fragmentation already encompasses a large number of different reactions and specific reaction mechanisms. For example, reactions such as substitution, rearrangement or elimination often occur in the context of fragmentation. The content presented here therefore does not cover the entire spectrum of the term coarse fragmentation, but serves as an introduction to the principle of this type of reaction.
Reaction principle
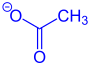
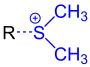
As already mentioned, in connection with coarse fragmentation, one speaks of the division of an organic compound into three fragments. The process is shown here using a 3-chloropropyl alkyl ether (if R is an alkyl radical) as an example. A resulting fragment leaves the ether without taking the binding electron pair with it. It is therefore usually present as a cation (on the product side in green ). In older, English-language literature, this fragment is also referred to as an electrofuge . Another fragment splits off as a neutral, unsaturated compound (on the product side in black ) from the starting compound, while a third fragment, usually in the form of an anion (on the product side in blue ), arises from the educt due to the entrainment of the binding electron pair. The last-mentioned fragment is the so-called nucleofuge in older English-language literature .
In order to facilitate understanding in accordance with the literature, the two English-language terms electrofuge and nucleofuge are used in the following .
General fragmentation reaction mechanism
From a mechanistical point of view, the example just given can be represented as follows, whereby the coloring of the bonds and other structures depends (as above) on the belonging to the respective fragment being created:
If the radical R shown here is a proton, there is the possibility, with elimination of hydrogen, that the electrofuge is also present as a neutral compound. For this purpose, the second line of the table and the fragmentation of di-tert-butylmethanol can be viewed below .
Examples of resulting fragments
Corresponding to the coloration introduced so far, the following table is intended to give an exemplary overview of the fragments formed depending on the specific structures present before the fragmentation. The possible nature of the radicals R, R 1 and R 2 results in accordance with the coloration according to the table.
| Exemplary structure (left) and emerging fragment ( electrofuge , right) shown in green |
Exemplary structure (left) and the resulting neutral fragment (right) shown in black |
Exemplary structure (left) and resulting fragment ( nucleofuge , right) shown in blue |
|||
Mechanistic examples of fragmentation
As already made clear, the coarse fragmentation can be understood as a type of reaction in which different types of chemical reactions coincide. In the following, three exemplary reactions serve to illustrate the application of the reaction principle of coarse fragmentation to certain chemical reactions. The individual structures are colored as usual.
Fragmentation of Di- tert -butylmethanol
A fragmentation reaction is described in the context of dehydration and the associated intramolecular rearrangement of an alkyl group of di- tert- butylmethanol:
Mechanistically speaking, the hydroxide ion is first split off as a nucleofuge from the alcohol ( 1 ) , after which the intramolecular alkyl group migration and thus the formation of a tertiary carbenium ion takes place. With the elimination of a smaller carbenium ion, the electrofuge ( 2 ), the formation of a neutral alkene ( 3 ) follows:
With elimination of a proton, 2 can also be converted into the alkene in the further course, so that in this case the electrofuge is also present as an uncharged particle at the end of the reaction.
Fragmentation of 2,3-dibromo-3-phenylpropanoic acid
One proposal for this reaction mechanism first describes the deprotonation of the acid derivative ( 4 ) using sodium hydrogen carbonate . This is followed by the splitting off of carbon dioxide, as electrofuge , and a bromine ion, as nucelofuge , the formation of cis -1-bromo-2-phenylethene .:
1,4-Elimination of 1,4-dibromocyclohexane with fragmentation
This elimination reaction, described by Grob in 1955, is identified in the literature as giving its name to the Grob fragmentation reaction type. With regard to the dibromide used, the reaction proceeds in quantitatively the same yield for both the cis and the trans isomer. After adding zinc, the compound ( 6 ) decomposes into zinc bromide and diallyl ( 7 ):
See also
Atomic economy
The atom economy of coarse fragmentation cannot be clearly classified. With regard to the reaction principle, the simple division of a compound into three fragments, the efficiency of the coarse fragmentation can be classified as very good, insofar as the fragmentation is only used for structure elucidation, for example. If the aim is to synthesize a single fragment by means of fragmentation, low molecular weight wastes such as carbon dioxide or halides can arise, which reduces the efficiency of the corresponding reactions. Accordingly, the atom economy should be assessed separately, depending on the reaction under consideration.
Individual evidence
- ^ Daniel Zerong Wang: Comprehensive Organic Name Reactions and Reagents . tape 1 . John Wiley & Sons, Inc., Hoboken, New Jersey 2009, ISBN 978-0-471-70450-8 , pp. 1279-1283 , doi : 10.1002 / 9780470638859.conrr197 .
- ↑ a b c d e f g h i j Cyril. A. Grob, Peter. W. Schiess: Heterolytic Fragmentation. A Class of Organic Reactions. In: Angewandte Chemie International Edition in English. Volume 6, No. 1, 1967, pp. 1-15, DOI: 10.1002 / anie.196700011 .
- ↑ a b c Peter Schiess: Cyril A. Grob (1917-2003): Fragmentation and inductance. In: Angewandte Chemie. Volume 116, No. 34, 2004, p. 4492, DOI: 10.1002 / anie.200461144 .
- ↑ Werner Scholze-Stubenrecht (Ed.): Duden. German universal dictionary. 8th edition, Bibliographisches Institut GmbH, Berlin, 2015, ISBN 978-3-411-05508-1 , p. 636.
- ↑ a b Cyril. A. Grob: Mechanisms and Stereochemistry of Heterolytic Fragmentation. In: Angewandte Chemie International Edition in English. Volume 8, No. 8, 1969, pp. 535-546, DOI: 10.1002 / anie.196905351 .
- ↑ a b c d Kathrin Prantz, Johann Mulzer : Synthetic Applications of the Carbonyl Generating Grob Fragmentation. In: Chemical Reviews. Volume 110, No. 6, 2010, pp. 3741-3766, DOI: 10.1021 / cr900386h .
- ^ A b c Frank C. Whitmore, EE Stahly: The Common Basis of Intramolecular Rearrangements. II. The Dehydration of Di-tert-butylcarbinol and the Conversion of the Resulting Nonenes to Trimethylethylene and Isobutylene. In: Journal of the American Chemical Society. Volume 55, No. 10, 1933, pp. 4153-4157, DOI: 10.1021 / ja01337a042 .
- ↑ a b Erling Grovenstein Jr., Donald E. Lee: The Stereochemistry and Mechanism of the Transformation of Cinnamic Acid Dibromide to β-Bromostyrene. In: Journal of the American Chemical Society. Volume 75, No. 11, 1953, pp. 2639-2644, DOI: 10.1021 / ja01107a025 .
- ^ A b Stanley J. Cristol, William P. Norris: Mechanisms of Elimination Reactions. IX. The Spontaneous Decomposition of Salts of β-Halo Acids. II. Trans-Cinnamic Acid Dibromide. In: Journal of the American Chemical Society. Volume 75, No. 11, 1953, pp. 2645-2646, DOI: 10.1021 / ja01107a026 .
- ↑ a b c C. A. Grob, W. Baumann: The 1,4-elimination with fragmentation. In: Helvetica Chimica Acta. Volume 38, No. 3, 1955, pp. 594-610, DOI: 10.1002 / hlca.19550380306 .