Hemoglobin C.
| Classification according to ICD-10 | |
|---|---|
| D58.2 | Other hemoglobinopathies
HbC heterozygosity / homozygosity |
| D57.2 | Doubly heterozygous sickle cell disease
HbSC disease |
| D56.8 | Other thalassemias
HbC-β thalassemia |
| ICD-10 online (WHO version 2019) | |
Hemoglobin C ( Hb C or HbC, crystalline Hb ) is a hemoglobin structural variant and thus belongs to the hereditary hemoglobinopathies . It is caused by a point mutation in the HBB gene, which codes for the β-globin chain of hemoglobin . Due to the HBB mutation c.19G> A, a basic lysine residue (E6K substitution) is found in the β-globin at amino acid position 6 instead of acidic glutamic acid .
Clinical significance
In terms of clinical relevance, a distinction must be made between HbC carrier or heterozygosity and HbC homozygosity. In the latter, both HBB genes have the HbC mutation. Patients with HbC homozygosity inherited a mutated allele from their mother and father . As a result, the hemoglobin in these consists predominantly (> 95%) of hemoglobin C. Due to the lower solubility of HbC compared to physiological hemoglobin A (HbA), HbC crystals form in the erythrocytes . These cells are sequestered in the spleen . HbC homozygotes usually show a mild hemolytic anemia due to the breakdown of erythrocytes . In addition to target cells, microspherocytes and crystal cells appear in the blood smear .
In the case of HbC carriers , one HBB allele does not show any mutation and the carriers therefore also produce physiological HbA in addition to HbC. These carriers are clinically silent - so they show no symptoms. Only slight cellular changes in the erythrocytes can z. B. be observed in the form of target cells. However, HbC carriers pass on the HbC allele to half of their offspring, so that clinically relevant combination forms can result depending on the mutations that may be present in the partner. In countries with a high allele frequency, there are therefore appropriate screening programs to detect asymptomatic carriers such. B. to recognize family planning .
However, a considerable disease value results from combinations with other hemoglobinopathies. Compound heterozygous constellations with structural variants of the β chain and β-thalassemias are of particular importance .
The most common and clinically relevant is the combination of the "sickle cell allele", also known as HbS, and HbC. This so-called HbSC disease is v. a. frequently in Africa, as the distribution areas of HbS and HbC overlap in West Africa . The clinical appearance resembles that of a clinical milder sickle cell disease , since HbC does not polymerize as easily as HbS. There are therefore z. B. less acute vascular occlusions . On the other hand, HbSC patients are more likely to have significant retinopathies , avascular necrosis of the bone, or priapism . Morphologically, target cells are shown in the smear. The sickle cells characteristic of sickle cell disease are rarely found in HbSC disease. Instead, there are so-called crystal cells , which do not occur in sickle cell disease.
In the case of HbC-β o thalassemia , the patient has inherited an HBB allele that does not produce β-globin (β o allele) in addition to the HbC allele . Therefore, as in HbC homozygosity, these patients predominantly produce hemoglobin C. In contrast, however, only one functional HBB gene is present, so that in addition to slight hemolysis, the classic features of β-thalassemia minor with distinct microcytosis and hypochromasia are associated.
Epidemiology
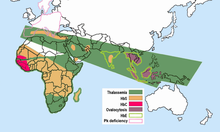
The causal mutation originated in West Africa. Since hemoglobin C confers relative protection against malaria , which is common in this region , HbC carriers offered a selection advantage . As a result, up to 25% of residents in West Africa are HbC carriers or have HbC homozygosity. The mutation came from these areas through the massive slave trade v. a. to America and the Caribbean. As a result, 2-3% of African Americans in the US have the HbC allele. Due to the slave trade, the HbS allele is also found relatively frequently in the present-day descendants of former slaves in the USA. As a result, HbSC disease occurs significantly more frequently than HbC homozygosity in the USA. After hemoglobin E and HbS, HbC is the third most common Hb structural anomaly worldwide. In Germany, however, HbC occurs relatively rarely. Clinically relevant combination forms are therefore correspondingly rare.
Individual evidence
- ↑ HbVar ID 227. Retrieved July 21, 2020 .
- ↑ Enno Kleihauer with the collaboration of Elisabeth Kohne and Andreas E. Kulozik: Anomalous hemoglobins and thalassemia syndromes: Basics and clinics . Ecomed, Landsberg 1996, ISBN 3-609-62760-3 .
- ^ Sickle cell and thalassaemia (SCT) screening: program overview. Accessed April 3, 2020 (English).
- ^ Lydia H. Pecker, Beverly A. Schaefer, Lori Luchtman-Jones: Knowledge insufficient: the management of hemoglobin SC disease . In: British Journal of Hematology . tape 176 , no. 4 , February 2017, p. 515-526 , doi : 10.1111 / bjh.14444 , PMID 27982424 , PMC 5303157 (free full text) - ( wiley.com [accessed April 3, 2020]).
- ^ RL Nagel, ME Fabry, MH Steinberg: The paradox of hemoglobin SC disease . In: Blood Rev . tape 17 , no. 3 , September 2003, p. 167-178 , doi : 10.1016 / S0268-960X (03) 00003-1 , PMID 12818227 .
- ↑ Orphanet: Hemoglobin C beta thalassemia syndrome. Retrieved April 3, 2020 .
- ↑ Steinberg, Martin H .: Disorders of hemoglobin: genetics, pathophysiology, and clinical management . 2nd ed. Cambridge University Press, New York 2009, ISBN 978-0-521-87519-6 .
- ↑ Mark A. Travassos, Drissa Coulibaly, Matthew B. Laurens, Ahmadou Dembélé, Youssouf Tolo: Hemoglobin C Trait Provides Protection From Clinical Falciparum Malaria in Malian Children . In: Journal of Infectious Diseases . tape 212 , no. 11 , December 1, 2015, ISSN 0022-1899 , p. 1778–1786 , doi : 10.1093 / infdis / jiv308 , PMID 26019283 , PMC 4633765 (free full text).
- ↑ RM Fairhurst, H. Fujioka, K. Hayton, KF Collins, TE Wellems: Aberrant development of Plasmodium falciparum in hemoglobin CC red cells: implications for the malaria protective effect of the homozygous state . In: Blood . tape 101 , no. 8 , April 2003, p. 3309-3315 , doi : 10.1182 / blood-2002-10-3105 , PMID 12480691 .
- ↑ Elisabeth Kohne, Enno Kleihauer: Hemoglobinopathies in Germany . In: Deutsches Aerzteblatt Online . February 5, 2010, ISSN 1866-0452 , doi : 10.3238 / arztebl.2010.0065 , PMID 20186311 , PMC 2828242 (free full text).