Polyoxometalates
Polyoxometalates (abbreviated POM ) are a group of substances that have polyatomic anions . These are made up of three or more transition metal oxyanions (such as vanadate or tungstate ) and bridged by oxygen atoms. You can form a large, closed, three-dimensional network.
The metal atoms are usually transition metals of groups 5 or 6 in high oxidation numbers , that is, their electronic configuration is d 0 or d 1 . Examples are vanadium (V), niobium (V), tantalum (V), molybdenum (VI), and tungsten (VI), with the largest number of the polyoxometalates known today being composed of the last two metals mentioned.
The polyoxometalates can be divided into two groups: heteropoly anions and isopoly anions. Heteropoly anions are metal clusters with enclosed hetero anions, such as sulfate or phosphate ions. Isopoly anions are pure metal oxide networks without heteroatoms. They are often less stable than their heteropolyanion counterparts. Together with acidic hydrogen ions, polyoxometalates form the heteropoly acids , which are used as catalysts.
presentation
The polymetalates can be prepared in two ways: On the one hand, by protonating an oxo ligand of the metal cation in acidic solution, which creates an H 2 O ligand that can be split off from the central metal atom and thus leads to condensation of the mononuclear oxometallates (cf. Fig.) And on the other hand by the condensation reaction of polyacids in the basic. Depending on the pH of the solution, polyoxometalate frameworks of different sizes are created.
Attachment behavior

Probably the best-known example of the condensation reaction between metal oxides is the formation of dichromate from two chromate anions, with the oxygen-bridged dichromate anion being formed with elimination of water. Long-chain, oxygen-bridged polychromates also form in very acidic solutions. However, the tendency to form such species is limited by the fact that the chromates only connect via the corners of their tetrahedra. A connection via edges and surfaces would lead to a convergence of the metal centers, which would be thermodynamically unfavorable for the chromium cation. Chromium (VI) prefers the coordination number 4, whereas some of its neighboring elements (in groups 5 and 6), such as molybdenum and tungsten, which are also in the + VI oxidation state, prefer six-fold coordination. Nevertheless, the smaller poly-molybdates / -wungstates mainly occur as tetrahedron-linked units. Larger structures then also contain the thermodynamically for z. B. molybdenum cheaper, octahedral coordinated metal cations. The conversion from tetrahedral to octahedral units can take place during the condensation; however, the units of the polyoxometalates can also be present as octahedra beforehand.
The structures of polyoxometalates can contain two types of bridging oxygen atoms, on the one hand the -MOM compound (reaction type: olation ) and the -M- (OH) -M compound (reaction type: oxolation ). The formation of polyoxometalates is most pronounced in groups 5 and 6 with vanadium (V), molybdenum (VI) and tungsten (VI) (due to their low 5th and 6th ionization energy and the ionic radius ).
The structural and chemical properties, as well as the stability of many polyoxometalates, are so varied that it is difficult to give a general and comprehensible description of their synthesis, even if there are some well-known species. It therefore often proves to be advantageous to depict the structures of the metallate ions as polyhedra. The metal cation is in the middle and the oxygen atoms at the corners (based on the basic units of the polyoxometalates, the tetra and octahedra). The polyspecies can result from a corner, edge or surface link. Among other things, the important M 6 O 19 structure of u. a. [Mo 6 O 19 ] 6- , as well as the M 7 O 24 structure of u. a. Represent [Mo 7 O 24 ] 2- by polyhedron structures.
The structures of the polyhedra (with heteroatoms) are mostly named after their discoverer, since other naming methods have proven to be complicated and difficult. The Keggin, Anderson and Dawson ions are worth mentioning at this point. So z. B. The Keggin structure is a hollow sphere made of 12MO 6 octahedra with the heteroatom in the center.
|

|
|
|
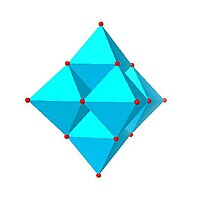
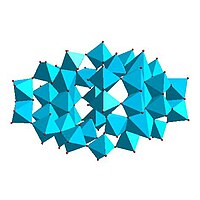
| Lindqvist hexamolybdate, | Decavanadate , | Paratungstate B, | Mo 36 -polymolybdate, |

|

|

|
|
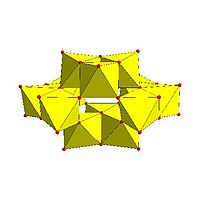
| Strandberg structure, | Keggin structure , | Dawson structure, | |
|

|

|

|
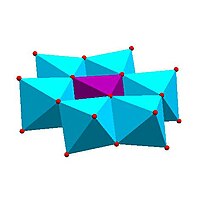
| Anderson structure, | Allman – Waugh structure, | Weakley – Yamase structure, | Dexter – Silverton structure, |
species
Polyoxovanadates
Polyoxovanadates (POVs) belong to the group of polyoxometalates (POMs), they generally consist of several [MO x ] units (with M = V, Nb, Mo, Ta, W and x = 4 - 7). Mostly they have an octahedral or square pyramidal coordination geometry. The metal centers are often in ad 0 or d 1 [electron configuration]. The POVs show a wide variety of species. There is also the possibility of incorporating heteroatoms into polyoxovanadate anions. Often some vanadium atoms in the structures are replaced by arsenic, antimony, germanium or silicon, for example, so that what are known as heteropoly anions are formed. It is also possible to bind organic molecules or transition metal complexes to the POVs. This results in new physical and chemical properties.
In contrast to their counterparts with molybdenum or tungsten, polyoxovanadates are often produced solvothermally . Many synthesis parameters are decisive for the formation of the polyoxovanadates. Among other things, the pH value, as individual species can transform into one another by changing the pH value. At a very high pH value, orthovanadate [VO4] 3- is predominantly present, which when acidified in polyvanadate, such as. B. decavanadate [V 10 O 28 ] 6- , skip over.
Isopolyacids and isopoly-metalates
So-called isopolyacids are inorganic polyacids that are formed by condensation of an acid. They have only one kind of central atom (mostly the subgroup elements vanadium, niobium, tantalum, chromium, molybdenum and tungsten, as well as the main group elements boron, silicon, phosphorus, arsenic and sulfur). Isopolyacids and isopoly-metalates are usually grouped together due to their structural similarities, whereby the metalates are, strictly speaking, the acid anion of the corresponding polyacid.
Examples
Individual evidence
- ↑ MT Pope: Isopolyanions and Heteropolyanions. In: Comprehensive Coordination Chemistry. Vol. 3, Pergamon Press, Oxford 1987, pp. 1023-1058.
- ↑ a b c Duward F. Shriver, Peter W. Atkins, Cooper Harold Langford, Wolfgang Kaim, Manfred Weidenbruch, Jürgen Heck, Detlef Rehorek, Brigitte Schwederski, Inis C. Tornieporth-Oetting, Gudrun Uhl: Inorganische Chemie. 2nd Edition. Wiley-VCH, Weinheim 1997, p. 321.
- ↑ Achim Müller, Erich Krickemeyer, Jochen Meyer, Hartmut Bögge, Frank Peters: [Mo154 (NO) 14O420 (OH) 28 (H2O) 70] (25 ± 5) -: A Water-Soluble Big Wheel with More than 700 Atoms and a Relative Molecular Mass of About 24000 . In: Angewandte Chemie International Edition in English . tape 34 , no. 19 , October 16, 1995, ISSN 0570-0833 , p. 2122-2124 , doi : 10.1002 / anie.199521221 .
- ↑ Michael Binnewies, Maik Finze, Manfred Jäckel, Peer Schmidt, Helge Willner, Geoff Rayner-Canham: Allgemeine und Anorganische Chemie. 3. Edition. Springer, Wiesbaden / Berlin / Heidelberg 2015, p. 681.
- ^ F. Albert Cotton, Geoffrey Wilkinson, Carlos A. Murillo, Manfred Bochmann: Advanced Inorganic Chemistry. 6th edition. Wiley-Interscience, New York 1999, pp. 930ff.
- ↑ Philipp Kurz, Norbert Stock: Synthetic Inorganic Chemistry: Basic Course. Walter de Gruyter, Berlin 2013, pp. 76–77.
- ^ Arnold F. Hollemann, Nils Wiberg: Textbook of Inorganic Chemistry. 101st edition. de Gruyter-Verlag, Berlin / New York 1995, p. 1464.
- ↑ Juan J. Borrás-Almenar, E. Coronado, Achim Müller, Michael Pope: Polyoxometalate Molecular Science. Springer Science & Business Media, Berlin / Heidelberg 2012, p. 34.
- ↑ Duward F. Shriver, Peter W. Atkins, Cooper Harold Langford, Wolfgang Kaim, Manfred Weidenbruch, Jürgen Heck, Detlef Rehorek, Brigitte Schwederski, Inis C. Tornieporth-Oetting, Gudrun Uhl: Inorganic Chemistry. 2nd Edition. Wiley-VCH, Weinheim 1997, pp. 321-322.
- ↑ De-Liang Long, Ryo Tsunashima, Leroy Cronin : Polyoxometalates as building blocks for functional nanosystems . In: Angewandte Chemie . tape 122 , no. 10 , 2010, p. 1780–1803 , doi : 10.1002 / anie.200902483 .
- ↑ Yoshihito Hayashi: Hetero and lacunary polyoxovanadate chemistry: Synthesis, reactivity and structural aspects . In: Coordination Chemistry Reviews . tape 255 , no. 19-20 , 2011, pp. 2270-2280 , doi : 10.1016 / j.ccr.2011.02.013 .
















