Solvothermal synthesis
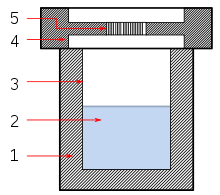
The solvothermal is a synthetic strategy in inorganic chemistry . For this purpose, the solvent with the starting materials is placed under high pressure (and high temperatures) in an autoclave . In such a solvent many substances dissolve better than under normal conditions , so that reactions are possible that would otherwise not take place. This makes it possible to display new connections or modifications . Solvothermal synthesis can also be viewed as a special case of a chemical transport reaction.
definition
Byrappa and Yoshimura defined the solvothermal synthesis as: "[...] any heterogeneous chemical reaction in the presence of a solvent (whether aqueous or nonaqueous) above room temperature at a pressure greater than 1 atm in a closed system." (Eng .: "[...] any heterogeneous chemical reaction that takes place in the presence of a solvent (be it aqueous or not) above room temperature and at a pressure greater than one atmosphere).
history
The first evidence of a successful solvothermal synthesis can be found in 1845 by Schafhäutl , who was able to obtain quartz crystallites from freshly precipitated silica in a Papin pot . In 1848, Bunsen succeeded in obtaining crystal needles of various carbonates with the help of thick-walled glass or baromether tubes and cooling an ammoniacal solution at 200 ° C under 15 bar pressure (this process is the forerunner of visual hydrothermal synthesis). The modern form of hydrothermal synthesis was invented by de Sénarmont in 1851, who used melted glass ampoules as a reaction vessel, which were placed in an autoclave to avoid explosions. Around 80 silicate minerals were synthesized by 1900 . The emergence of new alloys led to autoclaves lined with precious metals that suited new reaction media. Today one mainly uses the arrangement specified by Morey in 1914 (for work up to 400 ° C and 400 bar), the Tuttle - "cold seal" arrangement (1949; for work up to 900 ° C and 2000 bar when using alloys on cobalt or nickel-based) or the internally heated autoclave (with water-cooled steel jacket) developed by Smith and Adams in 1923, which is suitable for work at up to 1400 ° C and 10,000 bar.
Autoclaves
As a rule, the solvothermal synthesis requires the use of autoclaves. An autoclave is used to protect the reaction vessel, and the autoclave itself is often the reaction vessel. The Morey arrangement is best suited for work up to 400 ° C and 400 bar . This arrangement consists of an autoclave jacket in which the reaction vessel is located. The reaction vessel is placed in the lower part of the autoclave jacket and closed with a hexagonal closure part. The material of the jacket is often tool steel, it is possible to completely line the inside with precious metals so that the reactions can be carried out in the autoclave itself, and it is also possible to use ampoules as reaction vessels. The entire autoclave is in an oven and is heated.
The Tuttle "cold seal" autoclave consists of a steel cylinder in which a hole has been turned. This end is immersed in the oven, the screw cap with conical seal is outside the oven (often with water cooling to achieve a sufficiently low temperature). The pressure in the Tuttle "cold seal" autoclave must be applied externally, the reaction vessel is always an ampoule (often made of glass or a precious metal). This arrangement can be used up to 750 ° C and 5000 bar. The working range can be extended by using special alloys for the sheath (e.g. cobalt and nickel based alloys).
For most applications, the Tuttle and Morey arrangements work well enough. If higher temperatures are required for a synthesis, the heating must be installed inside a water-cooled steel jacket. This arrangement was first described by Smith and Adams in 1923 .
Materials
The material of the reaction vessel must have some important properties in order to be suitable for high temperature / pressure applications. It must be corrosion-resistant to the solvent used and the reaction conditions, and it must not contaminate the reaction products.
solvent
In addition to water (hydrothermal synthesis) as the most important solvothermal reaction medium, there are a large number of solvents used, including: methanol , ammonia (ammonothermal synthesis), carbon dioxide or glycols (e.g. with 1,6-hexanediol , called glycothermal synthesis).
Water as the reaction medium
The pVT data of water are well known up to 1000 ° C and 10 kbar. Important properties of water at high pressures and temperatures include complete dissociation into H 3 O + and OH - at around 150–200 kbar and 1000 ° C; the viscosity, which decreases with increasing temperature and pressure, and the dielectric constant decreasing with increasing temperature and increasing with increasing pressure .
Formic acid as the reaction medium
Formic acid decomposes at high temperatures into carbon dioxide and hydrogen or into carbon monoxide and water. The formic acid is thus transformed into a reducing and carbon dioxide-rich reaction medium in which it is possible to obtain numerous oxides and carbonates .
Ammonia as the reaction medium
The critical temperature of ammonia is 132.2 ° C and the critical pressure 111 bar. Under such conditions it is quite possible to obtain a whole range of amides , imides and nitrides . Even if the dielectric constant of ammonia is lower than that of water, it appears, especially at high pressures, as a polar medium.
use
Important areas of application for solvothermal synthesis are:
- The single crystal growth to the crystals to grow, the one for the structure determination by XRD have a sufficient size.
- The element synthesis. A large number of elements were hydrothermally obtained as a single crystal for the first time.
- The synthesis of metastable compounds, such as. B. monoclinic selenium in carbon disulfide .
- The oxide synthesis . Oxides of transition metals in unusual oxidation states are almost exclusively accessible by solvothermal synthesis.
- The chalcogenide synthesis . The production of anhydrous alkali metal chalcogenides is easily possible with ammonothermal means.
- The halide synthesis . Fluorides , fluoride hydroxides and oxides can be produced hydrothermally (with hydrofluoric solution in gold ampoules) .
- The synthesis of micro- or mesoporous compounds (e.g. zeolites or metal-organic framework compounds ).
- The synthesis of inorganic nanoparticles , such as nanoparticles of titanium (IV) oxide .
State of the art of solvothermal synthesis
| parameter | SiO 2 | GaN |
|---|---|---|
| Autoclave size | 0.65 m diameter
14 m length |
3 cm in diameter
20–70 cm in length |
| Autoclave volume | 4.6 m 3 | 0.0004 m 3 |
| Maximum yield per pass | 2.3 t | a few grams |
High throughput methods
High throughput methods (HD methods) are a sub-area of combinatorial chemistry and a tool for increasing efficiency. There are basically two synthesis strategies within the HD methods:
- With the combinatorial approach , all reactions take place in one vessel, which leads to product mixtures.
- In parallel syntheses, the reactions take place in different vessels.
High-throughput solvothermal syntheses are solvent-based.
High throughput methods for the expolative synthesis of MOFs
High throughput synthesis methods in discovering new, analog or isoreticular organometallic frameworks ( Engl . Metal organic frameworks , MOFs) used. A large number of reactions with different conditions (solvent and educt or additive ratios) are carried out in parallel and then the crystallinity and phase purity of the reaction products are examined. Special reactors are used for parallel synthesis (see also the section on HD methods in the article on organometallic frameworks). Syntheses that result in crystalline products are then repeated on a preparative scale and the products are further analyzed.
Other uses
- In computational chemistry, high-throughput methods are used, among other things, to test large substance libraries for various properties. Li et al. For example, in 2016 we selected over 5000 MOFs based on their ability to selectively adsorb CO 2 at high humidity and then performed high-throughput Grand Canonical Monte Carlo (GCMC) simulations with the 15 most suitable MOFs. Various structural databases are available for MOFs for simulation purposes, for example the " computation-ready, experimental (CoRE) MOF database ".
- As a screening method is that, in this case, then as a high-throughput screening (HTS) ( Engl . High-throughput screening (HTS)) referred to, procedures in the pharmacy used to screen large compound libraries for their biochemical , genetic or pharmacological Enable activity.
literature
- Albrecht Rabenau: The role of hydrothermal synthesis in preparative chemistry. In: Angewandte Chemie . 1985, 97, 12, pp. 1017-1032, doi : 10.1002 / anie.19850971205 .
- William S. Sheldrick, Michael Wachhold: Solvatothermal synthesis of chalcogenidometallates. In: Angewandte Chemie . 1996, 109, 3, pp. 214-234, doi : 10.1002 / anie. 19971090305
- Andreas Stein, Steven W. Keller, Thomas E. Mallouk : Turning Down the Heat: Design and Mechanism in Solid-State Synthesis. In: Science . 1993, 259, pp. 1558-1564, doi : 10.1126 / science.259.5101.1558 .
Individual evidence
- ↑ a b c d e f g h i j Albrecht Rabenau: The role of hydrothermal synthesis in preparative chemistry . In: Angewandte Chemie . tape 97 , no. 12 , December 1, 1985, ISSN 1521-3757 , pp. 1017-1032 , doi : 10.1002 / anie.19850971205 .
- ↑ a b c Dhanaraj, G., Byrappa, K., Prasad, V., Dudley, M. (Eds.): Springer Handbook of Crystal Growth . Springer, Heidelberg 2010, ISBN 978-3-540-74761-1 , p. 658 ff .
- ↑ a b c Sebastian Bauer, Norbert Stock: High-throughput methods in solid-state chemistry. Get there faster . In: Chemistry in Our Time . tape 41 , no. 5 , October 2007, ISSN 0009-2851 , p. 390–398 , doi : 10.1002 / ciuz.200700404 .
- ↑ ML Kelty, W. Morris, AT Gallagher, JS Anderson, KA Brown: High-throughput synthesis and characterization of nanocrystalline porphyrinic zirconium metal – organic frameworks . In: Chemical Communications . tape 52 , no. 50 , 2016, ISSN 1359-7345 , p. 7854–7857 , doi : 10.1039 / C6CC03264H ( rsc.org [accessed September 18, 2019]).
- ↑ Song Li, Yongchul G. Chung, Randall Q. Snurr: High-Throughput Screening of Metal-Organic Frameworks for CO2 capture in the Presence of Water . In: Langmuir . tape 32 , no. 40 , October 11, 2016, ISSN 0743-7463 , p. 10368-10376 , doi : 10.1021 / acs.langmuir.6b02803 .
- ^ D Cronk: Chapter 8 - High-throughput screening . In: Drug Discovery and Development (Second Edition) . Churchill Livingstone, 2013, ISBN 978-0-7020-4299-7 , pp. 95–117 , doi : 10.1016 / b978-0-7020-4299-7.00008-1 ( sciencedirect.com [accessed September 18, 2019]).
