Metal-organic framework compound
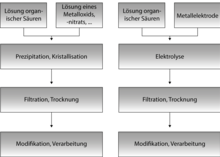
Metal organic frameworks or metal-organic frameworks ( English metal-organic frameworks , MOFs) are microporous materials consisting of inorganic structural units, the so-called SBUs (engl. Secondary building units , SBUs) and organic molecules (engl. Left ) as connection elements between the inorganic units are constructed. Metal-organic frameworks are often, but not necessarily, crystalline . MOFs are so-called coordination polymers (more precisely: coordination networks) with an open framework that contains possible pores. MOFs are usually based on Werner complexes . After synthesis, the pores of the three-dimensional structures are filled with guest molecules (e.g. solvents or unreacted linkers). By removing the guest molecules (e.g. by heating , in a vacuum or a combination of both), the pores can be made accessible under certain circumstances. Potential areas of application are in gas storage (e.g. hydrogen , methane ), material separation, sensor technology and catalysis .
presentation
Solvothermal syntheses
MOFs are usually prepared using solvothermal syntheses . Solvothermal syntheses take place in closed reaction vessels, at temperatures above the boiling point of the solvent and at high pressures. These conditions increase the solvency of the solvent, so that even poorly soluble substances can be used for the synthesis. Molecular building blocks can be used in solvothermal synthesis and complex, metastable products such as MOFs can be generated.
High throughput methods
High throughput methods (HD methods) are a sub-area of combinatorial chemistry and a tool for increasing efficiency. There are basically two synthesis strategies within the HD methods: On the one hand, the combinatorial approach, here all reactions take place in one vessel, which leads to product mixtures, and on the other hand, the parallel synthesis, here the reactions take place in different vessels. A further distinction is made between thin films and solvent-based processes.
Thin films are made by various evaporation methods, such as. B. Electron beam and thermal evaporation or chemical transport reactions are produced. Classic HD investigations within the solvent-based methods are solvothermal and sol-gel syntheses .
Solvothermal syntheses can be carried out classically in a Teflon reactor in a convection oven or in glass reactors in a microwave oven (high-throughput microwave oven, HDMW). The use of a microwave oven changes the reaction parameters that are necessary to arrive at the desired product, sometimes dramatically.
High throughput solvothermal syntheses in a Teflon reactor

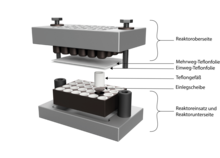
For high-throughput solvothermal syntheses, a solvothermal reactor with (e.g.) 24 cavities for Teflon reactors is used (see figure on the right). Such a reactor is sometimes referred to as a multiclav. The reactor block or reactor insert is made of stainless steel and contains 24 reaction chambers which are arranged in four rows. With the miniaturized Teflon reactors, volumes of up to 2 mL can be used. The reactor block is closed in a stainless steel autoclave; To do this, the filled reactor insert is inserted into the bottom of the reactor, the Teflon reactors are sealed with two Teflon sheets and the top of the reactor is put on. The autoclave is then closed in a hydraulic press. The sealed solvothermal reactor can then be subjected to a temperature-time program. The reusable Teflon film is used to withstand mechanical stress, while the disposable Teflon film seals the Teflon reactors. The sole use of disposable Teflon film means that it travels under the mechanical stress and the Teflon reactors run dry, the desired reaction cannot take place this way. After the reaction, the products can be isolated and washed in parallel in a vacuum filter device. The products are then separated on the filter paper in a so-called sample library and can then be characterized by automated X-ray powder diffraction. The information obtained is then used to plan further syntheses.
High-throughput solvothermal syntheses in a microwave oven
Filled with the reaction solution and a stir bar, a small glass reactor (e.g. with a volume of 2 mL) is closed with a Teflon cap and a screw cap. The vessels are placed in a reaction block made of silicon carbide, which among other things enables an even distribution of heat. Covered with a splash or explosion protection, the reaction block is positioned in the microwave oven, then a temperature-time program can be set. The products are isolated using a Teflon reactor as in the workflow.
Epitaxially growing MOFs - SURMOFs
Researchers at the Institute for Functional Interfaces (IFG) at KIT , Jacobs University Bremen and other institutions have developed a new process for the production of organometallic frameworks (MOFs) . Here, the MOF structures grow epitaxially , that is, in layers, on the surface of substrates (SURMOFs - Surface Mounted Metal Organic Frameworks). In this way, the size and shape of the pores as well as their chemical functionality can be tailored for the respective application. A special method, liquid phase epitaxy (LPE), also allows framework structures to be fabricated that cannot be created using normal wet-chemical methods. To produce this novel series of MOFs, known as SURMOFs 2, the scientists synthesized various organic molecules of different lengths. The pore size of the new organometallic framework is up to three by three nanometers. The researchers are working on increasing the length of the organic struts even further in order to embed even larger proteins and, in the next step, even metallic nanoparticles in the framework structures, which would enable interesting applications in optics and photonics.
Post-synthetic modification
Some MOFs cannot be synthesized because they are unstable in the possible reaction conditions. One possibility to represent these MOFs are postsynthetic modifications in which linkers and metal ions are exchanged after the actual synthesis. The postsynthetic exchange of linkers and metal ions is a growing area of research and therefore opens up opportunities for more complex structures and increased functionality.
Crystallinity and crystallization process
High temperatures and a high pressure inside the reaction vessel generally provide good conditions for crystal growth, since crystal defects (which MOFs tend to) can be resolved again. The crystallization can now be observed using real-time SEM .
Modulators and additives
Modulators and additives are substances that are supposed to support the formation of the MOF. In general, modulators can either facilitate the nucleation process or intervene in the particle growth. Modulators used in the manufacture of MOFs can be divided into two main groups, namely deprotonating and coordinating modulators. The former facilitate the binding of linkers to the metallic clusters by deprotonating linker molecules and accordingly accelerate MOF formation, while the latter compete with the linkers and reduce the rate of formation. Coordinating modulators are typically monocarboxylate molecules that compete with the polycarboxylate scaffolding linkers and result in differences in crystal size and morphology. For example, the use of formic acid in the synthesis of MIL-88A leads to larger particles with a more uniform size distribution with a simultaneous loss of morphology (diamond-shaped crystals become spherical).
Types
A meaningful classification of MOFs can be based on the linker molecules used.
Metal carboxylates
Metal carboxylates are organometallic framework compounds that are built up by the coordination of carboxylate ligands to metal ions. The availability, distribution and variety of linker molecules with deprotonatable acid groups has led to a large variety of metal-organic framework compounds.
synthesis
The synthesis is usually carried out under hydrothermal or solvothermal conditions with reaction times in the range from a few hours to several days. As is usual with MOFs, the discovery of new compounds involves the screening of various reaction parameters such as pH value , temperature, concentration, the amount and type of mineralizers or crystallization modulators (see "Presentation" above).

Linker molecules
Molecules containing carboxylate groups can be used as the anions of their acids as ligands of metal cations; molecules with two or more carboxylate groups are particularly frequently used .
Dicarboxylate linkers
The following molecules, among others, can be used as dicarboxylate linkers:
- Terephthalic acid (e.g. in MOF-5 or MIL-53 )
- 4,4′-sulfonyldibenzoic acid (e.g. in IITKGP-6 or CAU-11)
MOF-5 is considered to be one of the best studied MOFs, its simplified empirical formula is Zn 4 O (BDC) 3 . The inorganic structural unit consists of a Zn 4 O tetrahedron. An organic ligand (1,4-benzene dicarboxyl, BDC for short) binds to each edge of the tetrahedron, resulting in six BDC molecules on each Zn 4 O-SBU. The other ends of the BDC molecules bind to other Zn 4 O clusters. This creates a regular, cubic lattice in which the Zn 4 O clusters form the corner points of a cube and the BDC molecules form the edges. Molecules of the solvent used for synthesis remain in the cavities inside the cubes . The solvent is removed by heating.
Metal phosphonates
In addition to carboxylic acids, phosphonic acids can also be used to build up three-dimensional framework structures. Metal phosphonates (MPs) are organometallic framework compounds that are built up by the coordination of phosphonate ligands to metal ions. Due to the higher number of ligand atoms and the stronger charge in comparison to carboxylates, a higher chemical stability is to be expected. Due to the stronger CP bond compared to the CC bond, increased thermal stability can also be expected. But these are also the reasons why metal phosphonates tend to form dense chains or layered structures. They therefore also have a low solubility in many solvents.
Metal phosphonates are characterized by a very flexible and complex structural chemistry.
Metal phosphonate-based hybrid materials can also be used in a wide variety of areas - for example, for gas storage and separation, as a catalyst or as an ion exchanger, in intercalation chemistry and proton conduction.
synthesis
The synthesis of MPs is typically a time-consuming process for MOFs. It should be noted that each phosphonate system reacts uniquely and the behavior of the reaction mixture cannot be foreseen up to now.
Linker molecules
The anions of the following molecules can be used as phosphonate linkers:
- N, N -piperazine diphosphonic acid (e.g. in MIL-91 (Al) and MIL-91 (Ti))
- Phenylphosphonic acid ( ) (e.g. at )
history
Research on metal phosphonates was carried out in 1978 by Alberti and Costantino et al. justified. They made three Zirkoniumphosphonate ago: Zirkoniumphenylphosphonat, ; Zirkoniumhydroxymethylphosphonat, and Zirkoniumethylphosphat, .
Mixed functional ligands
Molecules with more than one type of functional group that can coordinate the metal center are often used to influence the properties of the MOF. Examples include:
- Functionalized porphyrin derivatives (so-called porphyrin-based MOFs, PMOFs for short)
- 2,2′-bipyridine-5,5′-dicarboxylic acid (e.g. the MOF Ga (OH) (bpydc), known as COMOC-4 has a MOF-253 topology and has luminescent properties)
structure

The structure of classic inorganic solids, such as B. the ionic compound sodium chloride or the metal copper , can usually be described with two competing but otherwise complementary concepts. One model is based on connected coordination polyhedra, the other on (closest) packing of spheres. However, these models are no longer applicable as soon as z. B. the degree of irregularities or the porosity increases. Zeolites are already characterized by rings of different sizes that form three-dimensional channels and cages. The way in which these rings are spatially connected is decisive for the description of important properties, such as the absorption behavior. The spatial connection of the rings is described in the network topology .
Since MOFs are made up of SBUs and linkers, there are a myriad of possibilities; it is therefore sensible to describe them using types of different networks (within the network topology).
Network topology
MOFs are described using networks. A network consists of the so-called nodes or connectors, which are connected via the linker. The nodes are the inorganic building blocks in the form of isolated metal atoms or metal-oxygen clusters. An easily accessible example is a honeycomb pattern , which represents a 6 3 network: Each node of a six-ring connects three further six-rings.
Nowadays there are a variety of methods used to describe the networks and this has created a great deal of confusion within research.
Among other things, the following are used:
- Schläfli symbols , including long Schläfli symbols
- Crystallographic point groups
- Vertex symbols
- Delaney symbols
- The three-letter code of the RCSR database
Structure of the inorganic building blocks
The inorganic building blocks can consist of isolated metal atoms, metal-oxygen clusters , chains or layers. Structural peculiarities here can be, for example, bridging hydroxide groups ( μ-OH) .
Breathing effect
When so-called. " Breathing effect " ( German effect of breathing, respiration) is the phenomenon that in each MOFs the crystallographic cell parameters as a function of external parameters change. This structural flexibility is an intensely researched property of MOFs. External parameters that can trigger breathing are temperature or pressure changes and the presence or absence of adsorbed guest molecules. Breathing does not break any covalent bonds, the overall topology of the MOF is retained, and all structural changes are reversible. Inner parameters that trigger breathing generally depend on:
- The type of metal cation in the chains
- The chemical nature of the various guest molecules;
- The strength of the host-guest interactions within the pores
In particular, the breathing effect can only occur if there are weak points in the framework. Three types of vulnerabilities are possible:
- Isomerization of the linker (e.g. with CAU-10-H or CAU-13).
- Rotation of the ligand around the OO axis of the carboxylate groups ( English kneecap mechanism , German kneecap mechanism , among other things to be observed in MIL-53).
- Shift of interwoven networks (see catenation ).
If only the cell volume changes, but not the other crystallographic cell parameters, we speak of "flexible structures" rather than "breathing".
Catenation
The catenation (from Latin catena , chain ') describes the mutual penetration of two or more networks, the porosity of the connection usually decreases. However, if there are strong interactions between the networks, they reduce their distance from one another, which leads to a stabilization of the connection with hardly any reduction in porosity.
nomenclature
For materials that belong to the class of porous metal-organic framework compounds, it has become established to use acronyms instead of the less meaningful empirical formulas. In addition to the acronym MOF, there is a large variety of other abbreviations, which often consist of the name of the research institution, the location of the same, company name or the ligand groups. So stands z. B. MIL for Matériaux de l'Institut Lavoisier, ZIF (= Zeolitic Imidazolate Frameworks ), IRMOF (= IsoReticular Metal-Organic Framework ), HKUST (= Hong Kong University of Science & Technology ), COF (= Covalent Organic Frameworks ), BAF (= BergAkademie Freiberg Framework ) MFU (= Metal – Organic Framework Ulm University ) TOF (= Thorium Organic Framework ) or CAU (= Christian Albrechts University). So not every MOF has to be called “MOF”. Some well-known MOF structures are MOF-5 , MOF-177, HKUST-1, MIL-53 , BAF-4, and MFU-1.
Differentiation from zeolites
In contrast to zeolites , i.e. inorganic crystals with pores of a similar size, MOFs are less temperature-resistant. However, it is expected that the multiple possibilities of organic chemistry will lead to a greater variety of materials than with zeolites, and the lower mass density is also advantageous for some applications.
Zeolites with pore sizes of more than 1 nm are rare, enantiomerically pure zeolites do not exist to this day.
Applications
The large internal surface area (up to over 4500 m² / g for MOF-177) is important for possible applications as catalysts . The pore size can be determined exactly via the size of the organic ligands, so that only reactants of a certain size fit into it. A high selectivity can be expected as a result.
Some MOFs have very good adsorption properties, which make them interesting for use in adsorption chillers . In an adsorption chiller, heat or cold is generated by adsorption or evaporation of a solvent. For this purpose, the system is divided into two subsystems, one containing a solvent (e.g. water) and the other the MOF. Both subsystems are connected by a connecting pipe with a valve, if the valve is opened, the solvent can evaporate and extract heat from the environment (evaporation enthalpy), while heat is released when the solvent is adsorbed on the MOF (adsorption enthalpy).
Hydrogen storage
Hydrogen has the highest energy density of all fuels. However, if hydrogen is not compressed, its volumetric energy density is very low, so that energy-intensive compression and liquefaction processes are required for the transport and storage of hydrogen.
Therefore, the development of new hydrogen storage methods that reduce the required storage pressure is an active research area.
MOFs stand out as materials for adsorptive hydrogen storage due to their high specific surface area and their surface-to-volume ratio as well as their modifiable structures.
Compared to an empty gas cylinder, a gas cylinder filled with MOF can store more hydrogen at a certain pressure, since hydrogen molecules adsorb on the surface of MOFs, and MOFs are free of dead volume. Since hydrogen adsorption is mainly based on physisorption, many MOFs exhibit completely reversible uptake and release behavior. No major activation barriers are required for the release of the adsorbed hydrogen. The hydrogen storage capacity of a MOF is limited by the liquid phase density of hydrogen, as the benefits of MOFs can only be achieved when the hydrogen is in its gaseous state.
The extent to which a gas can adsorb on the surface of a MOF depends on the temperature and pressure of the gas. In general, the adsorption increases with decreasing temperature and increasing pressure (until a maximum at (typically) 20 to 30 bar is reached, after which the adsorption capacity decreases again).
The US Department of Energy (DOE) has published a list of annual technical system goals for the storage of hydrogen in light fuel cell vehicles to guide researchers in this area (2017: 5.5 wt% per 40 g / L ). A benchmark material is MOF-177, in which 7.5 wt .-% hydrogen can be stored with a capacity of 32 g / L at 77 K and 70 bar. MOF-177 consists of [Zn 4 O] 6+ clusters and has a measured BET surface area of 4630 m 2 / g.
Design principles
The practical application of MOFs for hydrogen storage poses several challenges. For hydrogen adsorption close to room temperature, for example, the adsorption energy would have to be increased considerably.
MOFs with carboxylate linkers have received by far the most attention in research because:
- they easy to synthesize and
- are easy to deprotonate in situ ,
- the metal – carboxylate bond formation is reversible, which facilitates the formation of well-ordered crystalline MOFs and
- the ability of bridging bidentate coordination favors a high degree of order.
The most common transition metal ions used in carboxylate-based MOFs are Cu 2+ and Zn 2+ .
Be 12 (OH) 12 (BTB) 4 is the first successfully synthesized and structurally characterized MOF that consists of a light main group metal ion and has a high hydrogen storage capacity. However, the compound is too toxic to be of practical use. However, because of promising properties, significant efforts are being made to develop MOFs composed of other main group light metal ions.
catalysis
MOFs can be used as heterogeneous catalysts, due to their large surface area, the adjustable porosity and the diversity of the chemical composition.
Zeolites are already extremely useful in catalysis and have been implemented in commercial applications, but the structural diversity of zeolites is limited by the low variability in coordination (fewer than 200 zeolites are known). In contrast to this, MOFs show a great structural diversity due to more diverse coordination geometries, polytopic linkers, and auxiliary ligands (including F - , OH -, and H 2 O). Furthermore, it is difficult to obtain zeolites with pore sizes larger than 1 nm, which limits the catalytic applications of zeolites to relatively small organic molecules (typically no larger than xylenes ); in addition, zeolites can still not be obtained in enantiomerically pure form, which makes their use in catalytic asymmetric synthesis, e.g. B. for the pharmaceutical, agrochemical and fragrance industries, excludes. Enantiomerically pure chiral ligands and their metal complexes, on the other hand, have already been incorporated into MOFs to obtain efficient asymmetric catalysts.
Inclusion of catalytically active noble metal nanoparticles
In porous catalysts, coordinatively unsaturated metal ions ( coordinatively unsaturated metal sites , CUSs) are of great advantage because these sites enable strong interactions with trapped gases or the coordination of organic molecules. The inclusion of catalytically active noble metals can be achieved through a multi-stage process: First, free coordination sites are created in the pores of the MOF by removing auxiliary ligands; the introduction of a new auxiliary molecule then allows the coordination of anionic noble metal salts in the pores, and finally it is the precious metal cation is reduced.
The introduction of the new auxiliary molecule (engl. As grafting grafting ) denotes the coordination of functional groups is meant (as amino groups) of organic molecules to the free coordination sites at the metal center of the MOFs.
Functionalization of MIL-101
The chromium (III) terephthalate MOF with the designation MIL-101 (Cr 3 (F, OH) - (H 2 O) 2 O [(O 2 C) -C 6 H 4 - (CO 2 )] 3 · n H 2 O ( n ≈ 25)) has two types of mesoporous pores with diameters of approx. 29 and 34 Å, which are accessible through two microporous windows of approx. 12 and 16 Å. This results in very large BET and Langmuir surfaces (4100 m 2 g −1 ± 200 m 2 g −1 ; 5900 m 2 g −1 ± 300 m 2 g −1 ) and numerous potentially free coordination sites (up to 3, 0 mmol / g) on the chromium cation.
This makes it possible to functionalize MIL-101 first in order to then introduce catalytically active noble metal nanoparticles; this can be done as follows:
- Production of the free coordination sites (CUSs) by heating the terminal auxiliary ligand (water) in a vacuum. Since the terminal auxiliary ligand is directed towards the center of the cages / pores, so are the CUSs.
- Coordination of amines (such as ethylenediamine (ED)) to the coordinatively unsaturated sites of the chromium cation in the dehydrated MIL-101, resulting in ED-MIL-101. The ED molecules now point to the center of the pores.
- Encapsulation of precious metals in ED-MIL-101: The encapsulation process comprises the protonation of the amine groups in the pores (ED) with an aqueous HCl solution, the reaction of the positively charged ammonium surface groups with anionic precious metal salts (e.g. [PdCl 4 ] 2- , [PtCl 6 ] 2- and [AuCl 4 ] - ) by exchanging the chloride anions and finally reducing the noble metal cations with NaBH 4 . The corresponding elementary noble metal (approx. 1% by weight) is then located in the ED-MIL-101 pores.
ED-MIL-101 is stable up to approx. 200 ° C and therefore suitable as a possible catalyst for Knoevenagel condensation. Indeed, ED-MIL-101 shows a remarkably high activities (and selectivity) in the Knoevenagel condensation of benzaldehyde and ethyl cyanoacetate to give ethyl trans-α-cyanocinnamate. ED-MIL-101 can easily be isolated from the reaction suspension by filtration and reused without any significant loss of activity.
Other MOFs functionalized for catalysis
In another example, Pd nanoparticles embedded in a defective HKUST-1 scaffold enable the creation of tunable Lewis basic sites, therefore this Pd / MOF composite is able to undergo stepwise oxidation of benzyl alcohol and Knoevenag- Perform condensation.
characterization
The following methods, among others, are available for characterizing a MOF:
- Vibrational spectroscopy
- X-ray powder diffraction
- CHNS elemental analysis
- Thermogravimetry
- Sorption measurement
Special features
IR spectroscopy
If bridging hydroxide groups (μ-OH) appear in the inorganic building unit of the MOF , a sharp OH band can be observed at approx. 3500 cm −1 . If the bridging hydroxide group is also involved in hydrogen bonds, the band broadens (e.g. the case with CAU-11, where the layers of the MOF are held together by hydrogen bonds between bridging hydroxide ions and sulfonyl groups.)
literature
- Stefan Kaskel: Pores using a modular system. ( Memento of October 6, 2007 in the Internet Archive ) In: Nachrichten aus der Chemie. 53, April 2005, pp. 394-399.
- A. Breitruck, HE Hoster, RJ Behm: Short-range order in a metal-organic network. In: J. Phys. Chem. C. 113, 2009, pp. 21265-21268. (PDF; 7.2 MB) ( Memento from May 25, 2010 in the Internet Archive )
Web links
Individual evidence
- ↑ a b c d e Sebastian Bauer, Norbert Stock: MOFs - Organometallic framework structures. Functional porous materials . In: Chemistry in Our Time . tape 42 , no. 1 , February 2008, ISSN 0009-2851 , p. 12-19 , doi : 10.1002 / ciuz.200800434 .
- ↑ a b c Stuart R. Batten, Neil R. Champness, Xiao-Ming Chen, Javier Garcia-Martinez, Susumu Kitagawa: Terminology of metal – organic frameworks and coordination polymers (IUPAC Recommendations 2013) . In: Pure and Applied Chemistry . tape 85 , no. 8 , July 31, 2013, ISSN 1365-3075 , p. 1715-1724 , doi : 10.1351 / pac-rec-12-11-20 ( degruyter.com [accessed on 16 October 2018]).
- ↑ Ulrich Stoeck: New, porous organometallic framework compounds and organometallic coordination polymers: Representation, characterization and evaluation of their potential for gas storage and catalysis . Ed .: Chair for Inorganic Chemistry I at the Technical University of Dresden. Dresden June 2013, p. 5 .
- ^ IUPAC Provisional Recommendations on Metal-Organic Framework and Coordination Polymer Terminology - CrystEngComm Blog. Retrieved August 26, 2018 (American English).
- ↑ a b c Sebastian Bauer, Norbert Stock: High-throughput methods in solid-state chemistry. Get there faster . In: Chemistry in Our Time . tape 41 , no. 5 , October 2007, ISSN 0009-2851 , p. 390–398 , doi : 10.1002 / ciuz.200700404 .
- ↑ a b c d e f g Stefan Kaskel: The Chemistry of Metal-Organic Frameworks: Synthesis, Characterization, and Applications. Wiley, 2016, ISBN 978-3-527-69308-5 , pp. 6-7 .
- ↑ Enrica Biemmi, Sandra Christian, Norbert Stock, Thomas Bein: High-throughput screening of synthesis parameters in the formation of the metal-organic frameworks MOF-5 and HKUST-1 . In: Microporous and Mesoporous Materials . tape 117 , no. 1-2 , 2009, pp. 111-117 , doi : 10.1016 / j.micromeso.2008.06.040 .
- ↑ Jinxuan Liu, Binit Lukose, Osama Shekhah, Hasan Kemal Arslan, Peter Weidler, Hartmut Gliemann, Stefan Bräse, Sylvain Grosjean, Adelheid Godt, Xinliang Feng, Klaus Müllen, Ioan ‐ Bogdan Magdau, Thomas Heine, Christof Wöll: A novel series of isoreticular metal organic frameworks: realizing metastable structures by liquid phase epitaxy . In: Nature Scientific Reports 2, Article number: 921 . 2012, doi : 10.1038 / srep00921 .
- ↑ Sunirban That Hyunuk Kim, Kimoon Kim: Metathesis in Single Crystal: Complete and Reversible Exchange of Metal Ions Constituting the frameworks of Metal - Organic Frameworks . In: Journal of the American Chemical Society . tape 131 , no. 11 , March 25, 2009, ISSN 0002-7863 , p. 3814-3815 , doi : 10.1021 / ja808995d .
- ↑ a b c d Elham Bagherzadeh, Seyed Mojtaba Zebarjad, Hamid Reza Madaah Hosseini: Morphology Modification of the Iron Fumarate MIL-88A Metal-Organic Framework Using Formic Acid and Acetic Acid as Modulators . In: European Journal of Inorganic Chemistry . tape 2018 , no. 18 , May 11, 2018, ISSN 1434-1948 , p. 1909–1915 , doi : 10.1002 / ejic.201800056 .
- ^ A b Thierry Loiseau, Christophe Volkringer, Mohamed Haouas, Francis Taulelle, Gérard Férey: Crystal chemistry of aluminum carboxylates: From molecular species towards porous infinite three-dimensional networks . In: Comptes Rendus Chimie . tape 18 , no. December 12 , 2015, ISSN 1631-0748 , p. 1350-1369 , doi : 10.1016 / j.crci.2015.08.006 .
- ^ A b c Stefan Kaskel: The Chemistry of Metal-Organic Frameworks: Synthesis, Characterization, and Applications. tape 1 . Wiley, 2016, ISBN 978-3-527-69308-5 , pp. 11-12 .
- ↑ Franck Millange, Richard I. Walton: MIL-53 and Its Analogues Isoreticular: a Review of the Chemistry and Structure of a prototypical Flexible Metal-Organic Framework . In: Israel Journal of Chemistry . tape 58 , no. 9-10 , October 2018, pp. 1019-1035 , doi : 10.1002 / ijch.201800084 .
- ↑ a b Steffen Hausdorf, Jörg Wagler, Regina Moßig, Florian ORL Mertens: Proton and Water Activity-Controlled Structure Formation in Zinc Carboxylate-Based Metal Organic Frameworks . In: Journal of Physical Chemistry A . tape 112 , no. 33 , August 1, 2008, ISSN 1089-5639 , p. 7567-7576 , doi : 10.1021 / jp7110633 , PMID 18652430 .
- ↑ a b c d e Stephen JI Shearan, Norbert Stock, Franziska Emmerling , Jan Demel, Paul A. Wright, Konstantinos D. Demadis, Maria Vassaki, Ferdinando Costantino, Riccardo Vivani, Sébastien Sallard, Inés Ruiz Salcedo, Aurelio Cabeza, Marco Taddei : New Directions in Metal Phosphonate and Phosphinate Chemistry . In: Crystals . tape 9 , no. 5 , May 24, 2019, p. 270 , doi : 10.3390 / cryst9050270 .
- ^ A b Abraham Clearfield: Metal Phosphonate Chemistry . In: Progress in Inorganic Chemistry . John Wiley & Sons, Inc., Hoboken, NJ, USA 2007, ISBN 978-0-470-16648-2 , pp. 371-510 .
- ↑ Michael T. Wharmby, Gordon M. Pearce, John PS Mowat, John M. Griffin, Sharon E. Ashbrook: Synthesis and crystal chemistry of the STA-12 family of metal N, N′-piperazinebis (methylenephosphonate) s and applications of STA-12 (Ni) in the separation of gases . In: Microporous and Mesoporous Materials (= Metal Organic Frameworks ). tape 157 , July 15, 2012, ISSN 1387-1811 , p. 3–17 , doi : 10.1016 / j.micromeso.2011.12.003 .
- ↑ Michael T. Wharmby, John PS Mowat, Stephen P. Thompson, Paul A. Wright: Extending the Pore Size of Crystalline Metal Phosphonates toward the Mesoporous Regime by Isoreticular Synthesis . In: Journal of the American Chemical Society . tape 133 , no. 5 , February 9, 2011, ISSN 0002-7863 , p. 1266-1269 , doi : 10.1021 / ja1097995 .
- ^ Matthias J. Beier, Wolfgang Kleist, Michael T. Wharmby, Reinhard Kissner, Bertram Kimmerle: Aerobic Epoxidation of Olefins Catalyzed by the Cobalt-Based Metal Organic Framework STA-12 (Co) . In: Chemistry - A European Journal . tape 18 , no. 3 , 2012, ISSN 1521-3765 , p. 887-898 , doi : 10.1002 / chem . 201101223 .
- ↑ Alex Aziz, A. Rabdel Ruiz-Salvador, Norge C. Hernández, Sofia Calero, Said Hamad: Porphyrin-based metal-organic frameworks for solar fuel synthesis photocatalysis: band gap tuning via iron substitutions . In: Journal of Materials Chemistry A . tape 5 , no. 23 , 2017, ISSN 2050-7488 , p. 11894-11904 , doi : 10.1039 / C7TA01278K .
- ^ Ying-Ya Liu, Roel Decadt, Thomas Bogaerts, Karen Hemelsoet, Anna M. Kaczmarek: Bipyridine-Based Nanosized Metal – Organic Framework with Tunable Luminescence by a Postmodification with Eu (III): An Experimental and Theoretical Study . In: Journal of Physical Chemistry C . tape 117 , no. 21 , May 30, 2013, ISSN 1932-7447 , p. 11302-11310 , doi : 10.1021 / jp402154q .
- ^ A b Stefan Kaskel: The Chemistry of Metal-Organic Frameworks: Synthesis, Characterization, and Applications. tape 1 . Wiley, 2016, ISBN 978-3-527-69308-5 , pp. 127-128 .
- ↑ C. Livage, C. Egger, G. Férey: In: Chem. Mater. 11, 1999, pp. 1546-1550.
- ^ H. Hayashi, AP Côté, H. Furukawa, M. O'Keeffe, OM Yaghi: In: Nature Materials . 6, 2007, pp. 501-506.
- ↑ M. Eddaoudi, J. Kim, N. Rosi, D. Vodak, J. Wachter, M. O'Keeffe, OM Yaghi: In: Science . 295, 2002, pp. 469-472.
- ^ SS-Y. Chui, SM-F. Lo, JPH Charmant, AG Orpen, ID Williams: In: Science. 283, 1999, pp. 1148-1150.
- ↑ L. Zhao, C. Zhong: In: J. Phys. Chem. C. 113, 2009, pp. 16860-16862.
- ^ S. Hausdorf, W. Seichter, E. Weber, F. Mertens: Dalton Transactions. 2009, pp. 1107-1113.
- ↑ M. Tonigold, Y. Lu, B. Bredenkötter, B. Rieger, S. Bahnmüller, J. Hitzbleck, G. Langstein, D. Volkmer: In: Angew. Chem. Int. Ed. 48, 2009, pp. 7546-7550.
- ↑ KM Ok, J. Sung, G. Hu, RMJ Jacobs, D. O'Hare: In: J. Am. Chem. Soc. 130, 2008, pp. 3762-3763.
- ↑ Dominik Fröhlich, Evangelia Pantatosaki, Panagiotis D. Kolokathis, Karen Markey, Helge Reinsch: Water adsorption behavior of CAU-10-H: a thorough investigation of its structure-property relationships . In: Journal of Materials Chemistry A . tape 4 , no. 30 , July 26, 2016, ISSN 2050-7496 , p. 11859-11869 , doi : 10.1039 / c6ta01757f .
- ↑ a b c d National Research Council (US). Committee on Alternatives and Strategies for Future Hydrogen Production and Use., National Academy of Engineering., National Academy of Sciences (US): The hydrogen economy: opportunities, costs, barriers, and R & D needs . National Academies Press, Washington, DC 2004, ISBN 0-309-53068-7 .
- ↑ Ronneau, Claude .: Énergie, pollution de l'air et développement durable . Presses universitaires de Louvain, Louvain-la-Neuve 2013, ISBN 978-2-87558-171-6 .
- ^ Leslie J. Murray, Mircea Dincă, Jeffrey R. Long: Hydrogen storage in metal-organic frameworks . In: Chemical Society Reviews . tape 38 , no. 5 , 2009, ISSN 0306-0012 , p. 1294 , doi : 10.1039 / b802256a , PMID 19384439 (English).
- ↑ a b Alexander U. Czaja, Natalia Trukhan, Ulrich Müller: Industrial applications of metal-organic frameworks . In: Chemical Society Reviews . tape 38 , no. 5 , 2009, ISSN 0306-0012 , p. 1284 , doi : 10.1039 / b804680h .
- ^ DOE Technical Targets for Onboard Hydrogen Storage for Light-Duty Vehicles. Retrieved September 18, 2019 .
- ↑ K. Mark Thomas: Adsorption and desorption of hydrogen on metal – organic framework materials for storage applications: comparison with other nanoporous materials . In: Dalton Transactions . No. 9 , 2009, ISSN 1477-9226 , p. 1487 , doi : 10.1039 / b815583f .
- ↑ a b Kenji Sumida, Matthew R. Hill, Satoshi Horike, Anne Dailly, Jeffrey R. Long: Synthesis and Hydrogen Storage Properties of Be 12 (OH) 12 (1,3,5-benzenetribenzoate) 4 . In: Journal of the American Chemical Society . tape 131 , no. 42 , October 28, 2009, ISSN 0002-7863 , p. 15120-15121 , doi : 10.1021 / ja9072707 .
- ↑ Susumu Kitagawa, Ryo Kitaura, Shin-ichiro Noro: Functional Porous Coordination Polymers . In: Angewandte Chemie International Edition . tape 43 , no. 18 , 2004, ISSN 1521-3773 , p. 2334-2375 , doi : 10.1002 / anie.200300610 .
- ↑ a b c d e f Young Kyu Hwang, Do-Young Hong, Jong-San Chang, Sung Hwa Jhung, You-Kyong Seo: Amine Grafting on Coordinatively Unsaturated Metal Centers of MOFs: Consequences for Catalysis and Metal Encapsulation . In: Angewandte Chemie International Edition . tape 47 , no. 22 , May 19, 2008, pp. 4144-4148 , doi : 10.1002 / anie.200705998 .
- ↑ Ying Chuan Tan, Hua Chun Zeng: Lewis basicity generated by localized charge imbalance in noble metal nanoparticle-embedded defective metal – organic frameworks . In: Nature Communications . tape 9 , no. 1 , December 2018, ISSN 2041-1723 , p. 4326 , doi : 10.1038 / s41467-018-06828-4 , PMID 30337531 , PMC 6194069 (free full text).
- ↑ Nele Reimer, Helge Reinsch, A. Ken Inge, Norbert Stock: New Al-MOFs Based on Sulfonyldibenzoate Ions: A Rare Example of Intralayer Porosity . In: Inorganic Chemistry . tape 54 , no. 2 , December 24, 2014, ISSN 0020-1669 , p. 492-501 , doi : 10.1021 / ic502242j .