MOF-5
| Crystal structure | |||||||
|---|---|---|---|---|---|---|---|
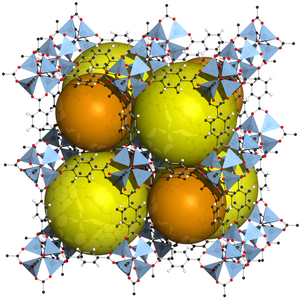
|
|||||||
| Eight cells of MOF-5, spheres to illustrate the pores present in the structure | |||||||
| General | |||||||
| Surname | MOF-5 | ||||||
| other names |
|
||||||
| Ratio formula | C 24 H 12 O 13 Zn 4 | ||||||
| External identifiers / databases | |||||||
|
|||||||
| properties | |||||||
| Molar mass | 769.99 g mol −1 | ||||||
| Physical state |
firmly |
||||||
| safety instructions | |||||||
|
|||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||
Along with HKUST-1 and MIL-53, MOF-5 (also IRMOF-1) is the best-known and best-studied MOF structure. It was first produced in 1999 by the group of Omar M. Yaghi (University of Michigan).
synthesis
Solvothermal synthesis
The synthesis of MOF-5 can be done in a number of ways. The best known is the solvothermal synthesis starting from zinc nitrate hexahydrate and terephthalic acid in N , N -diethylformamide , in which after 18 hours at temperatures of 90-105 ° C large crystals of the compound can be obtained, in the pores of which there are N , N ′ -diethylformamide as guest molecules. Exact investigations of the reaction have shown that the formation of the Zn 4 O-SBU is due to the decomposition of the NO 3 - anion in the solvent. Furthermore, a very low water content is important for the formation of MOF-5, since otherwise MOF-69c or zinc terephthalates are formed.
Subsequent oxide installation
Solvothermal synthesis is the most widely used for the preparation of MOF-5 with high porosity. The disadvantage, however, is the high consumption of N , N '-diethylformamide as a solvent. However, this can be drastically reduced by the method of subsequent oxide installation. The process includes two stages:
1. Precipitation of the usual zinc salt of the linker (here terephthalic acid, H 2 BDC) in water:
ZnCl 2 + Na 2 BDC + 2 H 2 O → ZnBDC · 2H 2 O ↓ + 2 NaCl
2. Implementation of the Salt with an oxide source while heating in suspension with N , N ′ -diethylformamide:
3 ZnBDC · 2H 2 O + Zn (NO 3 ) 2 · 4H 2 O → MOF-5 ↓ + 2 NO 2 + 0.5 O 2 + 10 H 2 O
The main advantage is that it is possible to work in highly concentrated suspensions instead of dilute solutions, which leads to an increase in the space-time yield to approx. 900% compared to the solvothermal process.
Inverse synthesis
In the inverse synthesis (also known as the “Controlled SBU Approach”), a complex compound is first produced which contains the Zn 4 O node , as is also contained in MOF-5. Suitable are e.g. B. the long-known complexes zinc oxoacetate [Zn 4 O (OOCCH 3 ) 6 ] or zinc oxobenzoate [Zn 4 O (OOCC 6 H 5 ) 6 ], which exchange their carboxylate ligands for other carboxylates in solution. In this way of ligand exchange, the acetate or benzoate ligands can be exchanged for terephthalate in solution , whereby MOF-5 and acetic acid or benzoic acid are formed. The choice of solvent is also crucial, since otherwise the terephthalic acid will protonate the oxo center of zinc oxoacetate or zinc oxobenzoate and destroy the node.
Base precipitation method
For the synthesis of microcrystalline MOF-5 particles, precipitation from a solution of zinc acetate dihydrate in N , N '-dimethylformamide with triethylamine and terephthalic acid is a convenient method. The material obtained in this way, however, has a lower porosity than MOF-5, which was produced in a solvothermal way.
Diffusion method
The first presentation was made by the slow diffusion of triethylamine into a solution of zinc nitrate hexahydrate and terephthalic acid in N , N ′ -dimethylformamide with chlorobenzene as a template.
structure
MOF-5 crystallizes in the cubic space group Fm 3 m (space group no. 225) . The structure can be derived from a cube on the eight corners of which the Zn 4 O nodes (SBU) are located and are linearly connected by terephthalate linkers. The center of the SBUs is an O 2− ion, which is tetrahedrally surrounded by four Zn 2+ ions. These are in turn bridged by the carboxylate groups of the terephthalate linker, so that an octahedral node with six surrounding terephthalates is created, which are arranged along the edges of the cube. For energetic reasons, the carboxy groups of the terephthalate ligands are in one plane with the aromatic ring. As a result, the nodes are arranged alternately in mirror image, which is associated with the formation of two pores of different sizes, since the aromatic rings of the terephthalate ligands are either oriented into the pore or out of it. The large pore has an opening of 13.8 Å and the small pore of 9.2 Å.
stability
MOF-5 is stable in dry air, but decomposes in moist air through contact with water. The structure is particularly sensitive to air humidity in the presence of DEF, since it is converted into MOF-69c. The conversion and decomposition is based on the protonation of the Zn 4 O oxo center. The solvent trapped in the pores as a result of the synthesis can be removed thermally without destroying the network. The thermal decomposition of MOF-5 takes place from approx. 320 ° C, with ZnO being formed last.
application
MOF-5 is under discussion as a material for gas storage, particularly for hydrogen and methane . The hydrogen storage capacity is 7.1 % by weight at 77 K and 40 bar; 10% by weight at 100 bar; corresponding to 66 g / l.
Analogues
In the meantime, main and sub-group analogues of MOF-5 have been synthesized by inverse synthesis. MOF-5 analogues based on beryllium and cobalt were produced in 2010 at the TU Freiberg.
literature
- Dipendu Saha, Shuguang Deng, Zhiguan Yang: Hydrogen adsorption on metal-organic framework (MOF-5) synthesized by the DMF approach . In: Journal of Porous Materials , 2009, 16 (2), pp. 141-149, doi: 10.1007 / s10934-007-9178-3 .
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ a b H. Li, M. Eddaoudi, M. O'Keeffe, O. Yaghi, Nature , 1999 , 402 , 276-279.
- ↑ NL Rosi, J. Eckert, M. Eddaoudi, DT Vodak, J. Kim, M. O'Keeffe, OM Yaghi, Science , 2003 , 300 , 1127-1129.
- ^ S. Hausdorf, J. Wagler, R. Moässig, FORL Mertens, J. Phys. Chem. A , 2008 , 112 (33) , 7567-7576, doi: 10.1021 / jp7110633 .
- ↑ S. Hausdorf, F. Mertens, F. Baitalow, J. Seidel, DE 10 2008 026 713 A1 "Process for the production of oxide-based metal-organic framework materials by means of reaction with incorporation of oxides".
- ^ S. Hausdorf, F. Mertens, F. Baitalow, J. Seidel, R. Fischer DE 10 2008 026 714 A1 "Process for the production of oxide-based metal-organic framework materials by means of inverse synthesis".
- ↑ DJ Tranchemontagne, JR Hunt, OM Yaghi, Tetrahedron , 2008 , 64 , 8553-8557.
- ↑ Steven S. Kaye et al .: Impact of Preparation and Handling on the Hydrogen Storage Properties of Zn 4 O (1,4-benzenedicarboxylate) 3 (MOF-5). J. Am. Chem. Soc. 129, 2007, doi: 10.1021 / ja076877g ( free full text ).
- ↑ Steffen Hausdorf, Felix Baitalow, Tony Böhle, David Rafaja, Florian ORL Mertens, J. Am. Chem. Soc., 2010, 132 (32), 10978-10981.