Julia olefination
The Julia olefination (also called Julia Lythgoe olefination ) is a name reaction in organic chemistry that was named after the French chemist Marc Julia after its discoverer . It is a chemical reaction of phenyl sulfones with aldehydes or ketones and is used to produce alkenes . The Julia- olefination is, among other methods, a standard process for producing carbon -Kohlenstoff- double bonds (carbonyl olefination).
mechanism
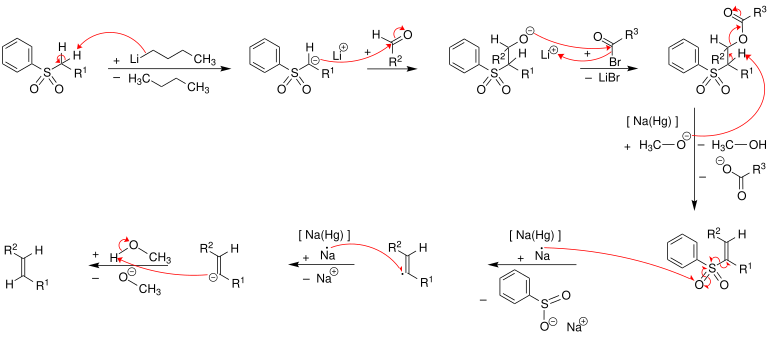
In the first step, the phenyl sulfone is deprotonated by butyllithium . The phenyl sulfone anion reacts nucleophilically with the carbonyl group of an aldehyde to form an alcoholate , which is esterified in a further step . The ester is eliminated to the alkene with the help of sodium amalgam [Na (Hg)] or samarium (II) iodide . The exact mechanism of the elimination is unknown - however, it can be assumed that the mechanism is a radical mechanism involving a vinyl radical . The following figure shows how the mechanism works according to the literature. All steps can be carried out as a one-pot reaction. This reaction gives preference to ( E ) -alkenes ( trans -alkenes).
The configuration of the alkene obtained does not depend on the configuration of the sulfone intermediate and therefore it is assumed that a thermodynamically stable ( E ) configuration can develop at the level of the vinyl radical .
variants
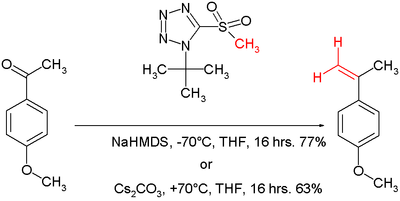
According to Philip Kocienski, a variant of the Julia reaction is known as the Julia-Kocienski olefination . The nucleophile here is a tetrazole . The mechanism is the same as in the Julia olefination. The reaction conditions can be varied between −70 ° C with sodium bis (trimethylsilyl) amide as base and +70 ° C with cesium carbonate as base in THF .
Individual evidence
- ↑ Marc Julia, Jean-Marc Paris: Syntheses a l'aide de sulfones v (+) - methode de synthesese generale de doubles liaisons. In: Tetrahedron Letters . tape 14 , no. 49 , 1973, pp. 4833-4836 , doi : 10.1016 / S0040-4039 (01) 87348-2 .
- ^ Gary E. Keck, Kenneth A. Savin, Michael A. Weglarz: Use of Samarium Diiodide as an Alternative to Sodium / Mercury Amalgam in the Julia-Lythgoe Olefination . In: The Journal of Organic Chemistry . tape 60 , no. 10 , May 1, 1995, pp. 3194-3204 , doi : 10.1021 / jo00115a041 .
- ↑ L. Kürti, B. Czakó: Strategic Applications Of Named Reactions In Organic Synthesis . Elsevier Academic Press, USA 2005, pp. 230 .
- ↑ Philip Kocienski: Recent sulphones-Based Reactions olefination . In: Phosphorus and Sulfur and the Related Elements . tape 24 , no. 1-2 , June 1, 1985, pp. 97-127 , doi : 10.1080 / 03086648508073398 .
- ↑ Sarah E. Kelly: 3.1 - Alkene Synthesis . In: Ian Fleming, Barry M. Trost (Eds.): Comprehensive Organic Synthesis . Pergamon, Oxford 1991, ISBN 978-0-08-052349-1 , pp. 729-817, here pp. 792-806 .
- ^ Paul R. Blakemore, William J. Cole, Philip J. Kocieński, Andrew Morley: A Stereoselective Synthesis of trans -1,2-Disubstituted Alkenes Based on the Condensation of Aldehydes with Metallated 1-Phenyl-1 H -tetrazol-5- yl sulfones . In: Synlett . tape 1998 , no. January 01 , 1998, p. 26-28 , doi : 10.1055 / s-1998-1570 .
- ↑ Christophe Aïssa: Improved Julia - Kocienski Conditions for the Methylenation of Aldehydes and Ketones . In: The Journal of Organic Chemistry . tape 71 , no. 1 , January 1, 2006, p. 360-363 , doi : 10.1021 / jo051693a .