Kiliani-Fischer synthesis
The Kiliani-Fischer synthesis (also cyanohydrin synthesis ) is used to extend the carbon chain of sugars , such as B. in the reaction of an aldopentose to aldohexose . It is named after Heinrich Kiliani and Emil Fischer .
Classic synthesis route
The main reaction path suggested by examining the intermediates in the course of the reaction provides D -arabinose → D -hexononitrile (a cyanohydrin ) → D -hexonoimidolactone → D -hexonolactone → D -hexonic acid.
First of all there is nucleophilic addition of hydrocyanic acid (HCN) to the carbonyl group of the aldose, here D -arabinose 1 . Since the attack on the carbon can occur from two sides, two diastereomeric cyanohydrins are formed , in this case 2a D- mannononitrile and 2b D -glucononitrile.
The nitrile then hydrolyses to form the carboxylic acid . This is initially a nucleophilic addition of water to the triple bond, followed by a rearrangement and finally a nucleophilic substitution , since the amino group is exchanged for a hydroxyl group . Two diastereomeric aldonates are formed: 3a D - mannonate and 3b D - gluconate .
In an acidic environment, spontaneous cyclodehydration occurs to five-membered lactones (γ-lactones): 4a γ- D - mannonolactone and 4b γ- D - gluconolactone .
The last step is the reduction of the carboxylic acid with sodium amalgam to the aldehyde. The sodium amalgam is oxidized to sodium amalgam oxide and two epimeric aldohexoses are ultimately formed : 5a D - mannose and 5b D - glucose .
Modern developments
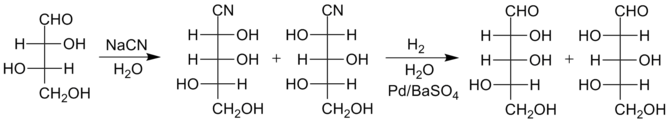
In 1958, R. Kuhn et al. a method which enables a higher yield especially for longer sugars such as glucose or mannose . Instead of converting the cyanohydrin to lactam, this is converted to the imine by means of reductive hydrogenation on Pd / BaSO 4 in water as a solvent. This in turn hydrolyzes instantly to the aldehyde under the given conditions. The use of poisoned catalysts, such as Lindlar catalysts , is necessary to prevent complete reduction to alcohol.
The example shows the chain extension from L - threose to L - xylose and L - lyxose .
Individual evidence
- ^ Heinrich Kiliani: About the cyanohydrin of the levulose . In: Reports of the German Chemical Society , Volume 18, Issue 2, pp. 3066-3072, 1885 , doi : 10.1002 / cber.188501802249 .
- ^ Emil Fischer, Reduction of acids of the sugar group, Reports of the German Chemical Society, Volume 22, Issue 2, pp. 2204–2205, 1889 , doi : 10.1002 / cber.18890220291 .
- ↑ Wang, Z .: Comprehensive Organic: Name Reactions and Reagents , Wiley Verlag, 2009, pp. 1613-1617, ISBN 978-0-471-70450-8 .
- ↑ Rajendra Varmaa, Dexter Frencha: Mechanism of the cyanohydrin (Kiliani-Fischer) synthesis . In: Carbohydrate Research . tape 25 , no. 1 , November 1972, doi : 10.1016 / S0008-6215 (00) 82748-2 .
- ↑ E. Broad Maier, G. Jung: Organic Chemistry , Georg Thieme Verlag, Stuttgart, 2005, ISBN 3-13-541505-8 .
- ↑ Richard Kuhn , Hans Grassner: Catalytic hydrogenation of hydroxy nitrites . In: Liebigs Annalen der Chemie , 612 , Heft 1, pp. 55-64, 1957 , doi : 10.1002 / jlac.19586120106 .