Lugdunin
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||
| General | ||||||||||
| Non-proprietary name | Lugdunin | |||||||||
| other names |
(1 R , 4 R , 7 R , 10 S , 13 R , 16 S , 19 R , 22 S ) -10- (1 H -indol-3-ylmethyl) -13-isobutyl-7,16,19,22 -tetraisopropyl-4- (sulfanylmethyl) -24-thia-3,6,9,12,15,18,21,26-octaazabicyclo [21.2.1] hexacosane-2,5,8,11,14,17,20 -hepton |
|||||||||
| Molecular formula | C 40 H 62 N 8 O 6 S | |||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| Drug information | ||||||||||
| Drug class | ||||||||||
| properties | ||||||||||
| Molar mass | 783.04 g · mol -1 | |||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
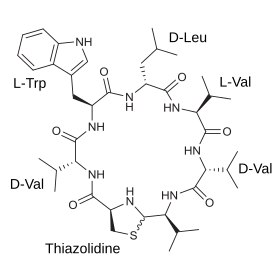
Lugdunin is a polypeptide antibiotic . It was isolated from Staphylococcus lugdunensis by a working group at the Eberhard Karls University of Tübingen and published in 2016. The bacteria come from samples taken from human noses . Lugdunin is the first known member of a new class of antibiotics, the authors macrocyclic thiazolidine - peptide called -antibiotics, and inhibits among other organisms, the growth of particularly aggressive multi-resistant pathogen Staphylococcus aureus USA300 .
structure
The unique Lugdunin structure consists of a ring of amino acid building blocks (a cyclic peptide ) in which the ring-shaped sulfur-nitrogen compound thiazolidine resides like a jewelry buckle (Latin: fibula). This is why the substance class is called fibupeptides. The thiazolidine ring is indispensable for the antibacterial effect.
Lugdunin can be obtained biotechnologically by extraction with 1-butanol from cultures of the bacterium S. lugdunensis . It is enriched in the organic phase and then purified by column chromatography . The chemical total synthesis , similar to the Merrifield synthesis , is also possible starting from the amino acids .
Many known peptide antibiotics bind to an enzyme due to their spatial structure and thus prevent the development of new bacterial cells. With Lugdunin, on the other hand, tests with its structural mirror image retained its antibiotic effect. Therefore, the effect is not based on spatial interaction.
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Alexander Zipperer, Martin C. Konnerth, Claudia Laux, Anne Berscheid, Daniela Janek, Christopher Weidenmaier, Marc Burian, Nadine A. Schilling, Christoph Slavetinsky: Human commensals producing a novel antibiotic impair pathogen colonization . In: Nature . 535, No. 7613, September, pp. 511-516. doi : 10.1038 / nature18634 .
- ↑ Nadine A. Schilling, Anne Berscheid, Johannes Schumacher, Claudia Steinem, Stephanie Grond: Synthetic Lugdunin Analogues Reveal Essential Structural Motifs for Antimicrobial Action and Proton Translocation Capability . In: Angewandte Chemie , May 6, 2019, doi: 10.1002 / anie.201901589 .