Lyxosis
| Structural formula | |||||||
|---|---|---|---|---|---|---|---|

|
|||||||
| Fischer projection , open-chain representation | |||||||
| General | |||||||
| Surname | D - (-) - lyxose, L - (+) - lyxose | ||||||
| other names |
|
||||||
| Molecular formula | C 5 H 10 O 5 | ||||||
| Brief description |
White dust |
||||||
| External identifiers / databases | |||||||
|
|||||||
| properties | |||||||
| Molar mass | 150.13 g mol −1 | ||||||
| Physical state |
firmly |
||||||
| Melting point |
108-112 ° C |
||||||
| solubility |
very soluble in water (586 g / l at 25 ° C, L- Lyxose) |
||||||
| safety instructions | |||||||
|
|||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||
Lyxose is a monosaccharide with five carbon atoms. This sugar belongs to the group of aldopentoses .
Occurrence
Both D- Lyxosis and L- Lyxosis do not occur naturally in free form, they are also only extremely rarely involved in the structure of cell membranes - as is the case with Mycobacterium phlei / Mycobacterium smegmatis , for example . Whenever “Lyxose” is mentioned in this text or in the scientific literature without any additional name ( prefix ), D- Lyxose is meant.
properties
The two cyclic constitutional isomers lyxofuranose and lyxopyranose are obtained through intramolecular hemiacetal formation . Since another center of asymmetry arises during hemiacetal formation , there are two diastereomers, α- and β-lyxofuranose, and α- and β-lyxopyranose. The α- and β-furanose or α- and β-pyranose pairs are also referred to as anomers .
In aqueous solution, the anomeric lyxofuranoses and lyxopyranoses are in equilibrium with one another via the unstable open-chain aldehyde form. The equilibrium is dominated to 71% by α-lyxopyranose, followed by β-lyxopyranose with 29%. The proportion of both Lyxofuranoses together is <1%. The establishment of equilibrium is called mutarotation .
D -Lyxosis - spellings Wedge formula Haworth notation 
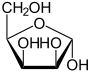
α- D -lyxofuranose
<1%
β- D -lyxofuranose
<1%
α- D -lyxopyranose
71%
β- D -lyxopyranose
29%
Individual evidence
- ↑ a b c d data sheet L- Lyxose at Sigma-Aldrich , accessed on December 21, 2019 ( PDF ).
- ↑ Data sheet L-Lyxose, 99% from AlfaAesar, accessed on January 11, 2020 ( PDF )(JavaScript required) .
- ↑ a b Data sheet D -Lyxose from Sigma-Aldrich , accessed on December 21, 2019 ( PDF ).
- ↑ KH Khoo, R. Suzuki, A. Dell, HR Morris, MR McNeil, PJ Brennan, GS Besra: Chemistry of the lyxose-containing mycobacteriophage receptors of Mycobacterium phlei / Mycobacterium smegmatis. In: Biochemistry. Volume 35, Number 36, September 1996, pp. 11812-11819, doi : 10.1021 / bi961055 + , PMID 8794763 .
- ↑ Eberhard Breitmeier, Günther Jung: Organic chemistry . Basics, substance classes, reactions, concepts, molecular structure. 5th edition. Georg Thieme Verlag, Stuttgart 2005, ISBN 3-13-541505-8 , p. 851 ( limited preview in Google Book search).