Methyl hypochlorite
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
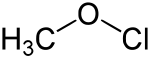
|
||||||||||
| General | ||||||||||
| Surname | Methyl hypochlorite | |||||||||
| Molecular formula | CH 3 ClO | |||||||||
| Brief description |
(below boiling point) yellow liquid |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 66.48 g mol −1 | |||||||||
| Physical state |
gaseous |
|||||||||
| density |
1.1 g cm −3 |
|||||||||
| boiling point | ||||||||||
| Refractive index |
1.343 (-20 ° C) |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||
Methyl hypochlorite is the ester of hypochlorous acid and methanol . It is unstable and can explode releasing toxic fumes. The instability of the compound is caused by the oxidative properties of the hypochlorite group . It is a source of chlorine in the earth's atmosphere .
Manufacturing
Esterification of hypochlorous acid with methanol :
It is also formed in the higher atmosphere through the reaction of methyl peroxyl radicals CH 3 OO with chlorine monoxide .
safety instructions
Methyl hypochlorite is an unstable, highly flammable, explosive substance. The compound is an oxidizing agent and as such is oxidizing. Upon contact with water, the substance decomposes according to the esterification reaction. Thus, when inhaled, corrosive vapors are generated which attack the alveoli. Methyl hypochlorite also decomposes at high temperatures in the following reactions:
Individual evidence
- ↑ Traugott Sandmeyer: About ethyl and methyl hypochlorite . In: Reports of the German Chemical Society . tape 19 , no. 1 , 1886, p. 857-861 , doi : 10.1002 / cber.188601901196 ( PDF ).
- ^ A b Carl L. Yaws: The Yaws Handbook of Physical Properties for Hydrocarbons and Chemicals Physical Properties for More Than 54,000 Organic and Inorganic Chemical Compounds, Coverage for C1 to C100 Organics and Ac to Zr Inorganics . Gulf Professional Publishing, 2015, ISBN 978-0-12-801146-1 , pp. 6 ( limited preview in Google Book search).
- ↑ a b Emil Abderhalden (Ed.): Biochemisches Handlexikon . Volume I, 1st half carbon, hydrocarbons, alcohols of the aliphatic series, phenols. Springer, 1911, ISBN 978-3-642-90817-0 , pp. 383 , urn : nbn: de: 1111-201202261673 .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ MJ Krisch, LR McCunn, K. Takematsu, LJ Butler, FR bladder, J. Shu: Photodissociation of CH 3 OCl to CH 3 at O + Cl 248 nm. In: The Journal of Physical Chemistry A. 108, 2004 S . 1650, doi : 10.1021 / jp0372082 .
- ↑ Ulrich Platt, Jochen Stutz: Differential Optical Absorption Spectroscopy Principles and Applications . Springer Science & Business Media, 2008, ISBN 978-3-540-75776-4 , p. 71 ( limited preview in Google Book search).
- ^ Ian Priestley, Linda Young, Robert Mullins and Phillip Brown, Syngenta Process Hazards Group, Huddersfield, UK: THE THERMAL STABILITY OF ALKYL HYPOCHLORITES. (No longer available online.) IChemE, 2011, archived from the original on May 20, 2016 ; accessed on May 22, 2016 (English). Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.





