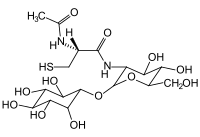
Mycothiol
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||
| General | |||||||||||||
| Surname | Mycothiol | ||||||||||||
| Molecular formula | C 17 H 30 N 2 O 12 S | ||||||||||||
| Brief description |
White dust |
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 486.50 g mol −1 | ||||||||||||
| Physical state |
firmly |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Mycothiol (MSH or AcCys-GlcN-Ins) is a bacterial thiol that occurs in actinobacteria and acts analogously to glutathione in eukaryotes. Mycothiol is used by the bacteria to break down reactive oxygen species and to detoxify them , including some antibiotics .
It consists of a cysteine component with an acetylated amino group attached to glucosamine . Again, this is linked to inositol . The oxidized disulfide form of mycothiol (MSSM) is called mycothion, which is reduced to mycothiol by the flavoprotein mycothion reductase.
Mycothiol biosynthesis enzymes and mycothiol-dependent enzymes such as mycothiol-dependent formaldehyde dehydrogenase and mycothion reductase are being taken as target substances for the development of treatments for tuberculosis .
Web links
- The Biochemistry of Pathogens: The metabolism and function of mycothiol in the mycobacteria. ( Memento from December 24, 2012 in the web archive archive.today )
Individual evidence
- ↑ K. Ajayi, S. Knapp, RC Lapo, VV Thakur: Intramolecular α-Glucosaminidation: Synthesis of Mycothiol. In: Org. Letters . Volume 12, 2010, pp. 2630-2633, doi: 10.1021 / ol1008334 .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ F. supporter, MW Vetting, PA Frantom, JS Blanchard: Structures and mechanisms of the biosynthetic enzymes Mycothiol. In: Current opinion in chemical biology. Volume 13, Number 4, October 2009, pp. 451-459, doi: 10.1016 / j.cbpa.2009.07.018 . PMID 19699138 . PMC 2749902 (free full text).
- ↑ RC Fahey: Novel thiols of prokaryotes . In: Annu. Rev. Microbiol. tape 55 , 2001, p. 333-356 , doi : 10.1146 / annurev.micro.55.1.333 , PMID 11544359 .
- ↑ VK Jothivasan, CJ Hamilton: Mycothiol: synthesis, biosynthesis and biological functions of the major low molecular weight thiol in actinomycetes. In: Natural Product Reports . Volume 25, 2008, pp. 1091-1117, doi: 10.1039 / B616489G
- ^ RC Fahey: Glutathione analogs in prokaryotes. In: Biochimica et Biophysica Acta . Volume 1830, number 5, May 2013, pp. 3182-3198, doi: 10.1016 / j.bbagen.2012.10.006 . PMID 23075826 .
- ^ GL Newton, N. Buchmeier, RC Fahey: Biosynthesis and functions of mycothiol, the unique protective thiol of Actinobacteria. In: Microbiology and molecular biology reviews: MMBR. Volume 72, Number 3, September 2008, pp. 471-494, doi: 10.1128 / MMBR.00008-08 . PMID 18772286 . PMC 2546866 (free full text).
- ^ GL Newton, N. Buchmeier, RC Fahey: Biosynthesis and functions of mycothiol, the unique protective thiol of Actinobacteria . In: Microbiol. Mol. Biol. Rev. Band 72 , no. 3 , September 2008, p. 471-494 , doi : 10.1128 / MMBR.00008-08 , PMID 18772286 , PMC 2546866 (free full text).
- ↑ MP Patel, JS Blanchard: Expression, purification, and characterization of Mycobacterium tuberculosis mycothione reductase . In: Biochemistry . tape 38 , no. 36 , September 1999, p. 11827-11833 , doi : 10.1021 / bi991025h , PMID 10512639 .
- ^ MP Patel, JS Blanchard: Mycobacterium tuberculosis mycothione reductase: pH dependence of the kinetic parameters and kinetic isotope effects . In: Biochemistry . tape 40 , no. 17 , May 2001, p. 5119-5126 , doi : 10.1021 / bi0029144 , PMID 11318633 .
- ↑ M. Rawat, Y. Av-Gay: Mycothiol-dependent proteins in actinomycetes . In: FEMS Microbiol. Rev. Band 31 , no. 3 , April 2007, p. 278-292 , doi : 10.1111 / j.1574-6976.2006.00062.x , PMID 17286835 .
- ^ GL Newton, RC Fahey: Mycothiol biochemistry . In: Arch. Microbiol. tape 178 , no. 6 , December 2002, p. 388-394 , doi : 10.1007 / s00203-002-0469-4 , PMID 12420157 .