N -cyclohexyl- N '- (2-morpholinoethyl) carbodiimide-methyl- p -toluenesulfonate
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
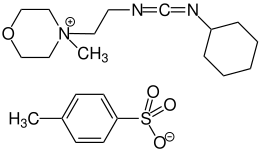
|
|||||||||||||
| General | |||||||||||||
| Surname | N -cyclohexyl- N '- (2-morpholinoethyl) carbodiimide-methyl- p -toluenesulfonate | ||||||||||||
| other names |
|
||||||||||||
| Molecular formula | C 14 H 26 N 3 O • C 7 H 7 O 3 S | ||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 423.57 g mol −1 | ||||||||||||
| Physical state |
firmly |
||||||||||||
| Melting point |
113-115 ° C |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
N -Cyclohexyl- N '- (2-morpholinoethyl) carbodiimide-methyl- p -toluenesulfonate is a coupling reagent from the group of carbodiimides . It is used in peptide synthesis . It was developed in 1956 by John Sheehan and Joseph Hlavka.
Individual evidence
- ↑ a b Data sheet N-Cyclohexyl-N ′ - (2-morpholinoethyl) carbodiimide methyl-p-toluenesulfonate, 95% from Sigma-Aldrich , accessed on October 22, 2016 ( PDF ).
- ↑ Horst Kunz, Regina Barthels: The 2- (2-pyridyl) ethoxycarbonyl (Pyoc) residue - an acid- and base-stable, hydrophilic protective group for the amino function in peptide synthesis. In: Angewandte Chemie . 95, 1983, p. 799, doi : 10.1002 / anie.19830951011 .
- ^ JC Sheehan, JJ Hlavka: Water soluble carbodiimide. In: J. Org. Chem. (1956), Vol. 21, p. 439.