Sodium laurate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
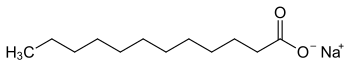
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Sodium laurate | |||||||||||||||
| other names |
Sodium dodecanoate |
|||||||||||||||
| Molecular formula | C 12 H 23 NaO 2 | |||||||||||||||
| Brief description |
yellowish odorless solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 222.30 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
242-244 ° C |
|||||||||||||||
| solubility |
easily soluble in water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Sodium laurate is the sodium salt of lauric acid .
properties
Sodium laurate is a flammable, difficult to ignite, yellowish odorless solid that is easily soluble in water.
use
Sodium laurate is used as an anionic surfactant . It is used in food as a binding agent, emulsifier and anti-caking agent.
Individual evidence
- ↑ a b c d e f Entry on sodium laurate in the GESTIS substance database of the IFA , accessed on January 9, 2019(JavaScript required) .
- ↑ NA Bergell, NA Chwala, NA Dittmer, NA Freytag, NA Gäbler, NA Gerhardt, NA Heyer, NA Hueter, NA Kehren, NA Koehler, NA Krings, NA Kröper: Soaps and soap-like substances . Springer-Verlag, 2013, ISBN 978-3-7091-5418-2 , pp. 598 ( limited preview in Google Book search).
- ↑ Shmuel Yannai: Dictionary of Food Compounds with CD-ROM, Second Edition . CRC Press, 2012, ISBN 978-1-4200-8352-1 , pp. 608 ( limited preview in Google Book search).

