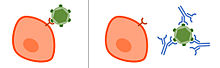
Neutralizing antibody
Neutralizing antibodies (NAb) are antibodies that have a neutralizing effect on the biological activity of a pathogen or a toxin .
properties
Neutralizing antibodies usually bind to proteins in the surface of a cell of the pathogen (in bacteria and fungi ) or on the virus surface in viruses and either sterically prevent the pathogen from binding to the host cell or prevent the conformational change of the proteins, which is necessary for entry is in the host cell. Thus, the antibodies can prevent the infection and possible damage by the pathogen without having to recruit cells from the immune system. Only some of the antibodies that are formed after infection or vaccination and bind to the pathogen have a neutralizing effect. Non-neutralizing antibodies bind to the pathogen, but do not have a neutralizing effect, but use other functions of antibodies , such as opsonization and activation of the complement system , to remove the pathogen.
Neutralizing antibodies are an important component of post-infection immunity , which protects against re-infection (sterilizing immunity). Neutralization is one of the three possible functions of an antibody. In the case of the immunoglobulin A subtype, neutralization is the main function. Passive immunization vaccines contain high concentrations of neutralizing antibodies. Typical examples of neutralizing antibodies are the diphtheria antitoxin , which is formed after vaccination with a diphtheria vaccine , as well as many other antitoxins . In the treatment of multiple sclerosis with interferon-beta , neutralizing antibodies can arise against it, which reduce the effectiveness of the treatment.
The neutralizing effect of antibodies can be measured with a neutralization test. A hemagglutination inhibition test and an animal experiment with passive immunization and subsequent infection ( stress test ) provide evidence of a neutralizing effect.
Broadly neutralizing antibodies
When developing vaccines against pathogens with a high genetic variability and, accordingly, many escape mutants , attempts are made to induce the formation of antibodies against as many variants, subtypes or strains of a pathogen as possible, e.g. B. broadly neutralizing anti-HIV antibodies and broadly neutralizing anti-IAV antibodies . Broadly neutralizing antibodies are being investigated for the dengue virus , the hepatitis C virus and the West Nile virus . The generation of broadly neutralizing antibodies by transfection with an AAV vector against HIV and the influenza virus (IAV) is also being investigated.
The bNAber database lists broadly neutralizing antibodies.
Individual evidence
- ↑ Susanne Modrow: Molecular Virology. Springer-Verlag, 2010, ISBN 978-3-827-42241-5 , p. 108.
- ↑ Susan Payne: Viruses. Academic Press, 2017, ISBN 978-0-128-03110-0 , p. 69.
- ↑ AL Schmaljohn: Protective antiviral antibodies that lack neutralizing activity: precedents and evolution of concepts. . In: Current HIV Research . 11, No. 5, July 2013, pp. 345-53. doi : 10.2174 / 1570162x113116660057 . PMID 24191933 .
- ↑ Christine Schütt, Barbara Bröker: Basic knowledge of immunology. Springer-Verlag, 2011, ISBN 978-3-827-42647-5 , p. 54.
- ↑ Hans W. Doerr: Medical Virology. Georg Thieme Verlag, 2010, ISBN 978-3-131-13962-7 , p. 136.
- ^ A. Bertolotto: Evaluation of the impact of neutralizing antibodies on IFNβ response. In: Clinica Chimica Acta . Volume 449, September 2015, pp. 31-36, doi : 10.1016 / j.cca.2015.02.043 , PMID 25769291 .
- ^ KL Hefferon: Broadly neutralizing antibodies and the promise of universal vaccines: where are we now? In: Immunotherapy. Volume 6, number 1, 2014, pp. 51-57, doi : 10.2217 / imt.13.150 , PMID 24341884 .
- ↑ C. Fauvelle, CC Colpitts, ZY Keck, BG Pierce, SK Foung, TF Baumert: Hepatitis C virus vaccine candidates inducing protective neutralizing antibodies. In: Expert review of vaccines. Volume 15, number 12, 12 2016, pp. 1535–1544, doi : 10.1080 / 14760584.2016.1194759 , PMID 27267297 , PMC 5156833 (free full text).
- ↑ J. Cohen: Immunology. Bound for glory. In: Science. Volume 341, number 6151, September 2013, pp. 1168–1171, doi : 10.1126 / science.341.6151.1168 , PMID 24030996 .
- ^ BC Schnepp, PR Johnson: Adeno-associated virus delivery of broadly neutralizing antibodies. In: Current opinion in HIV and AIDS. Volume 9, number 3, May 2014, pp. 250-256, doi : 10.1097 / COH.0000000000000056 , PMID 24638019 , PMC 4117238 (free full text).
- ↑ MP Limberis, VS Adam, G. Wong, J. Gren, D. Kobasa, TM Ross, GP Kobinger, A. Tretiakova, JM Wilson: Intranasal antibody gene transfer in mice and ferrets elicits broad protection against pandemic influenza. In: Science Translational Medicine . Volume 5, number 187, May 2013, p. 187ra72, doi : 10.1126 / scitranslmed.3006299 , PMID 23720583 , PMC 4596530 (free full text).
- ↑ AM Eroshkin, A. LeBlanc, D. Weekes, K. Post, Z. Li, A. Rajput, ST Butera, DR Burton, A. Godzik: bNAber: database of broadly neutralizing HIV antibodies. In: Nucleic acids research. Volume 42, Database issue January 2014, pp. D1133 – D1139, doi : 10.1093 / nar / gkt1083 , PMID 24214957 , PMC 3964981 (free full text).