Niobium oxide trichloride
| Crystal structure | |||||||
|---|---|---|---|---|---|---|---|
|
|||||||
| General | |||||||
| Surname | Niobium oxide trichloride | ||||||
| other names |
|
||||||
| Ratio formula | NbOCl 3 | ||||||
| Brief description |
colorless solid |
||||||
| External identifiers / databases | |||||||
|
|||||||
| properties | |||||||
| Molar mass | 215.3 g mol −1 | ||||||
| Physical state |
firmly |
||||||
| density |
3.05 g cm −3 |
||||||
| solubility |
|
||||||
| safety instructions | |||||||
|
|||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||
Niobium oxide trichloride is an inorganic chemical compound of niobium from the group of oxychlorides .
Extraction and presentation
Niobium oxide trichloride can be produced by reacting niobium (V) chloride with oxygen at 150 ° C
or obtained by hydrolysis.
It is also possible to produce it by reacting niobium (V) oxide with thionyl chloride at 200 ° C.
Or through the reaction of niobium (V) oxide and niobium (V) chloride:
Heated niobium (V) oxide in a stream of chlorine at 800–850 ° C also gives niobium oxide trichloride:
properties
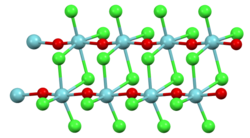
Niobium oxide trichloride is in the form of colorless needles. Above 350 ° C the decomposition into niobium (V) chloride and niobium (V) oxide begins. Like the corresponding yellow niobium oxide tribromide and black niobium oxide triiodide , it is very sensitive to moisture. In older literature, a tetragonal crystal structure with the space group P 4 2 / mnm (space group No. 136) , a = 1087 pm ; c = 396 pm, assumed, with recent investigations showing a non-centrosymmetric space group P 4 2 1 m (No. 113) (omission of the mirror planes perpendicular to the double strands). In addition to this oxychloride of niobium, niobium oxide dichloride is also known.
Individual evidence
- ↑ a b c d e f Georg Brauer: Handbook of preparative inorganic chemistry . 3., reworked. Edition. tape III . Enke, Stuttgart 1981, ISBN 3-432-87823-0 , pp. 1457 .
- ↑ a b c d Roger Blachnik (Ed.): Paperback for chemists and physicists . Volume III: Elements, Inorganic Compounds and Materials, Minerals . founded by Jean d'Ans, Ellen Lax. 4th, revised and revised edition. Springer, Berlin 1998, ISBN 3-540-60035-3 , pp. 632 ( limited preview in Google Book search).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Christian Kusterer: REACTIONS, STRUCTURES AND PROPERTIES OF OXIDE HALOGENIDES AND HALOOXOMETALLATES OF NIOB AND TUNGSTEN ( Memento of the original from November 12, 2013 in the Internet Archive ) Info: The archive link was automatically inserted and not yet checked. Please check the original and archive link according to the instructions and then remove this notice. . (PDF; 3.4 MB), 2006 (dissertation).




