Norrish reaction
Norrish reactions are name reactions from the field of organic chemistry . It is a photochemical cleavage of carbonyl compounds such as ketones or aldehydes . They are divided into two types called the Norrish Type I Response and the Norrish Type II Response . The reaction was first published in 1932 by the English chemist Ronald George Wreyford Norrish (1897–1978) and named after him. UV radiation of a specific wavelength is required to generate the radicals .
There are few preparative uses for the Norrish reactions. Norrish type I occurs as an undesirable side reaction in the Paternò-Büchi reaction and as a side reaction of Norrish type II. Both types are important secondary reactions in the photooxidative degradation of polymers .
Norrish Type I Reaction
Since the Norrish reaction is a reaction with reactive radical intermediates, which can continue to react with low selectivity , several reaction paths are possible.
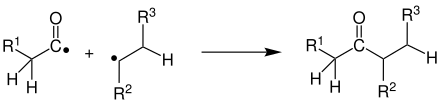
The reaction begins with the absorption of a photon by the carbonyl function , creating a radical in the singlet state . A triplet state can be formed from this by intersystem crossing (ISC). The extent to which this step takes place depends on the reactivity of the singlet radical and the speed of the intersystem crossing. However, both states lead to radical cleavage of the carbon-carbon bond or carbon- hydrogen bond on the carbonyl group. Which of the two radicals the carbonyl radical remains attached to depends on the stability of the alkyl radical.
The radical pair formed can now react in different ways:
- Recombination to the starting compound:
- Splitting off of carbon monoxide and subsequent combination of radicals:
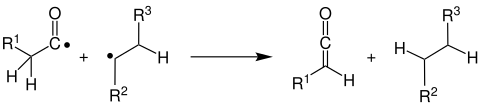
- The alkyl radical splits off a hydrogen radical from the carbonyl radical, creating a ketene and an alkane :
- The carbonyl radical splits off a hydrogen radical from the alkyl radical, creating an aldehyde and an alkene :
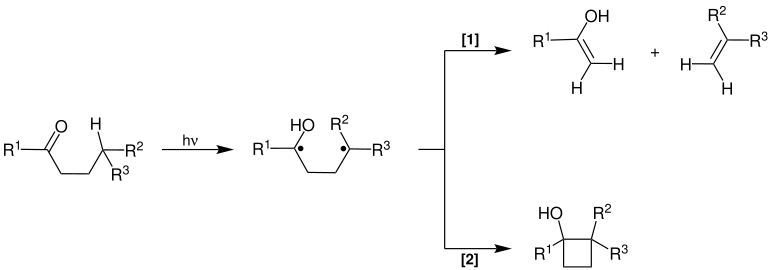
Norrish Type II Reaction
The Norrish reaction type II can take place with compounds which have a hydrogen atom in the γ position. It also begins with the photochemical generation of a biradical. In this case, however, a hydrogen radical in the γ position can be abstracted via a six-membered transition state, with a biradical alcohol being formed. This intermediate can now react in two ways:
- Fragmentation to an enol and an alkene [1]
- Combination to form a derivative of cyclobutane [2]
Web links
- Entry on Norrish Type I photoreaction . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.N04219 Version: 2.3.1.
- Entry on Norrish Type II photoreaction . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.N04218 Version: 2.3.1.
Individual evidence
- ^ RGW Norrish, FW Kirkbride: Primary photochemical processes. Part I. The decomposition of formaldehyde , in: J. Chem. Soc. 1932 , pp. 1518-1530; doi : 10.1039 / JR9320001518 .
- ↑ M. Day, DM Wiles: Photochemical degradation of poly (ethylene terephthalate). III. Determination of decomposition products and reaction mechanism . In: Journal of Applied Polymer Science . tape 16 , no. 1 , 1972, p. 203–215 , doi : 10.1002 / app.1972.070160118 .
- ↑ a b T. Laue, A. Plagens: Name and keyword reactions. , 4th edition, pp. 246-252, Teubner, Wiesbaden 2004, ISBN 3-519-33526-3 .
- ^ I. Ernest: Binding, Structure and Reaction Mechanisms in Organic Chemistry , Springer-Verlag, 1972, p. 332, ISBN 3-211-81060-9 .