Ombitasvir
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
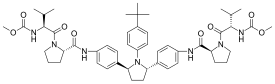
|
||||||||||||||||
| General | ||||||||||||||||
| Non-proprietary name | Ombitasvir | |||||||||||||||
| other names |
Dimethyl N , N '- {[(2 S , 5 S ) -1- (4- tert -butylphenyl) pyrrolidine-2,5-diyl] -bis - {[(4,1-phenyleneazanediyl) carbonyl] [(2 S ) -pyrrolidine-2,1-diyl]} [(2 S ) -3-methyl-1-oxobutane-1,2-diyl]} biscarbamate ( IUPAC ) |
|||||||||||||||
| Molecular formula | C 50 H 67 N 7 O 7 | |||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| Drug information | ||||||||||||||||
| ATC code | ||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 894.11 g mol −1 | |||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Ombitasvir is an antiviral drug for the treatment of hepatitis C sold by Gilead Sciences . It is used in combination with paritaprevir , ritonavir and dasabuvir as Viekira Pak against the hepatitis C virus genotype 1, and with paritaprevir and ritonavir under the name Technivie for the treatment of HCV genotype 4.
Ombitasvir is an NS5A inhibitor .
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ VIEKIRA PAK ™ (ombitasvir, paritaprevir and ritonavir tablets; dasabuvir tablets), for oral use. Full prescribing information . AbbVie Inc., North Chicago, IL 60064. Retrieved July 30, 2015.
- ↑ FDA approves Viekira Pak to treat hepatitis C . Food and Drug Administration . 19th December 2014.
- ↑ TECHNIVIE ™ (ombitasvir, paritaprevir and ritonavir) tablets, for oral use. Full prescribing information . AbbVie Inc., North Chicago, IL 60064. Archived from the original on January 19, 2019. Retrieved July 28, 2015.
- ↑ FDA approves Technivie for treatment of chronic hepatitis C genotype 4 . Food and Drug Administration . July 24, 2015.
- ↑ Jordan J. Feld, Kris V. Kowdley, Eoin Coakley, Samuel Sigal, David R. Nelson, Darrell Crawford, Ola Weiland, Humberto Aguilar, Junyuan Xiong, Tami Pilot-Matias, Barbara DaSilva-Tillmann, Lois Larsen, Thomas Podsadecki, Barry Bernstein: Treatment of HCV with ABT-450 / r – Ombitasvir and Dasabuvir with Ribavirin . In: N Engl J Med . 370, 2014, pp. 1594-1603. doi : 10.1056 / NEJMoa1315722 .