Oxime ester
| Oxime ester |
 General structure of the oxime esters of carboxylic acids. Here, R 1 to R 3 are organyl radicals (alkyl radicals, aryl radicals, arylalkyl radicals, etc.) or hydrogen atoms. If R 1 or R 2 represent a hydrogen atom, it is an aldoxime ester. The oxime ester group is marked in blue . When R 1 and R 2 are different, there are ( E , Z ) isomers. |
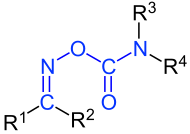 General structure of the oxime esters of carbamic acids . R 1 to R 4 are organyl residues (alkyl residues, aryl residues, arylalkyl residues, etc.). If R 1 or R 2 represent a hydrogen atom, it is an aldoxime carbamic acid ester. The oxime carbamic acid ester group is marked in blue . When R 1 and R 2 are different, there are ( E , Z ) isomers. |
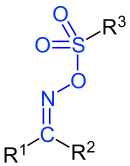 General structure of the oxime esters of sulfonic acids . Here, R 1 to R 3 are organyl radicals (alkyl radicals, aryl radicals, arylalkyl radicals, etc.). If R 1 or R 2 represent a hydrogen atom, it is an aldoxime sulfonic acid ester. The oxime sulfonic ester group is marked in blue . When R 1 and R 2 are different, there are ( E , Z ) isomers. |
Oxime esters are organic chemical compounds that are derived from oximes with the functional group C = N − OH, in that the hydrogen atom of the hydroxyl group is substituted by an acyl radical or a sulfonyl radical.
Manufacturing
Oxime esters are formed from oximes ( aldoximes or ketoximes ) and carboxylic acids with elimination of water. The oximes can also be acylated with carboxylic acid anhydrides , carboxylic acid chlorides or ketene . Aryl sulfonic acid esters of oximes can be prepared from sulfonic acid chlorides and oximes in pyridine or from sulfonic acid chlorides and the sodium salts of the oximes. Carbamic acid esters of aldoximes can be synthesized by reaction with carbamic acid chlorides or isocyanates .
Individual evidence
- ↑ Horst Metzger: Production and conversion of oximes in Houben-Weyl (editors: Eugen Müller, Otto Bayer, Hans Meerwein and Karl Ziegler): Methods of Organic Chemistry, Volume X / 4, Nitrogen Compounds I, Part 4, 1968, p. 1 -308, there pp. 180-181.
- ↑ Horst Metzger: Production and conversion of oximes in Houben-Weyl (editors: Eugen Müller, Otto Bayer, Hans Meerwein and Karl Ziegler): Methods of Organic Chemistry, Volume X / 4, Nitrogen Compounds I, Part 4, 1968, p. 1 -308, there pp. 182-184.
- ↑ Horst Metzger: Production and conversion of oximes in Houben-Weyl (editors: Eugen Müller, Otto Bayer, Hans Meerwein and Karl Ziegler): Methods of Organic Chemistry, Volume X / 4, Nitrogen Compounds I, Part 4, 1968, p. 1 –308, there p. 187.