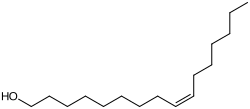
Palmitoleyl alcohol
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Palmitoleyl alcohol | |||||||||||||||
| other names |
cis -9-hexadecenol |
|||||||||||||||
| Molecular formula | C 16 H 32 O | |||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 240.42 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Palmitoleyl alcohol is a chemical compound from the group of unsaturated fatty alcohols . It is an alkenol with 16 carbon atoms and a cis -configured double bond at position C-9. The corresponding alcohol with the trans configuration is palmitelaidyl alcohol .
Occurrence
Palmitoleyl alcohol occurs in the form of its acetate as an insect pheromone.
Extraction and presentation
Palmitoleyl alcohol can be obtained by high pressure hydrogenation of methyl palmitoate .
Individual evidence
- ↑ a b c data sheet Palmitoleyl alcohol, ≥98% (capillary GC) from Sigma-Aldrich , accessed on May 21, 2015 ( PDF ).
- ↑ Martin Jacobson: Insect Sex Pheromones . Elsevier, 2012, ISBN 978-0-323-15241-9 , pp. 218 ( limited preview in Google Book search).
- ↑ Entry on palmitoleyl alcohol. In: Römpp Online . Georg Thieme Verlag, accessed on May 26, 2015.