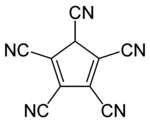
Pentacyanocyclopentadiene
| Structural formula | |||||||
|---|---|---|---|---|---|---|---|
|
|||||||
| General | |||||||
| Surname | Pentacyanocyclopentadiene | ||||||
| Molecular formula | C 10 HN 5 | ||||||
| External identifiers / databases | |||||||
|
|||||||
| properties | |||||||
| Molar mass | 191.1 g mol −1 | ||||||
| safety instructions | |||||||
|
|||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||
Pentacyanocyclopentadiene is a cyclic carbon compound . It contains five cyano groups (–CN) and two double bonds in the molecule , which is why it belongs to the cycloalkadienes .
The cyano group is also the functional group of this compound. Due to the relatively non-polar character of pentacyanocyclopentadiene, it is not soluble in water. The five cyano groups and the formation of an aromatic system (as in the parent compound cyclopentadiene ) stabilize the anion (by dissociating the H + ) in such a way that it is an extremely strong acid.
It has an extremely low pKa value of <−11. Such a value does not reach a mineral acid such as sulfuric acid (pK s value = -3.0), pentacyanocyclopentadiene therefore, by definition, to the Super acids is counted.
swell
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
literature
Vianello R et al. (2004): In search of ultrastrong Brønsted neutral organic superacids: a DFT study on some cyclopentadiene derivatives. Chemistry 10 (22); 5751-60; PMID 15484200
