Phenyl ester
Phenyl esters are chemical compounds that belong to the group of carboxylic acid esters . They are created by esterifying a carboxylic acid with phenol (PhOH). They have the characteristic functional ester group . In a broader sense, the phenyl esters also include carbonic acid esters and esters derived from sulfonic acids and phenol. Similarly, phenyl esters in the broader sense can also form from nitric acid , sulfinic acids , phosphonic acids or phosphinic acids on the one hand and phenol on the other.
Manufacturing
Phenyl esters arise z. B. from a carboxylic acid and phenol with acid-catalyzed dehydration . Phenyl esters can also be obtained from carboxylic acid chlorides and phenol by the Schotten-Baumann method . Phenyl esters can also be synthesized from carboxylic acid anhydrides and phenol .
Reactions
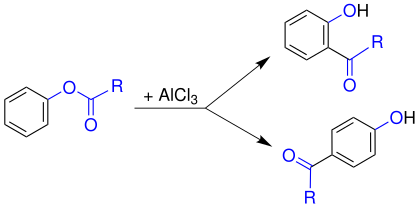
When exposed to a Lewis acid (e.g. aluminum chloride) at higher temperatures, phenyl esters are subject to the Fries rearrangement . In this form ortho - and para -hydroxy-phenyl ketones:
Individual evidence
- ↑ Eberhard Breitmaier, Günther Jung: Organische Chemie , 7th edition, Thieme Verlag, 2012, p. 353, ISBN 978-3-13-541507-9 .
- ↑ Eberhard wide Maier, Günther Jung: Organic Chemistry , 7th edition, Thieme Verlag, 2012, p 354, ISBN 978-3-13-541507-9 .
- ^ Siegfried Hauptmann : Organic Chemistry , 2nd revised edition, VEB Deutscher Verlag für Grundstoffindindustrie, Leipzig 1985, ISBN 3-342-00280-8 , p. 322.