Remimazolam
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
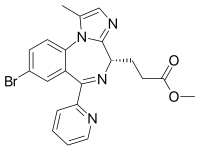
|
||||||||||||||||
| General | ||||||||||||||||
| Non-proprietary name | Remimazolam | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 21 H 19 BrN 4 O 2 | |||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| Drug information | ||||||||||||||||
| ATC code | ||||||||||||||||
| Drug class | ||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 439.31 g mol −1 | |||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Remimazolam is a drug from the group of benzodiazepines . Remimazolam was approved in Japan (as Anerem ) in January 2020 for general sedation and in the United States (as Byfavo ) in July 2020 for brief procedural sedation (for procedures lasting up to 30 minutes).
It is used parenterally in the form of the benzene sulfonic acid salt, remimazolambesylate .
Pharmacological properties
The mechanism of action is similar to that of other benzodiazepines : Remimazolam binds to GABA A receptors in the brain. Like other benzodiazepines, Remimazolam does not show any clear selectivity within the subtypes of the GABA A receptor. The primary metabolism takes place via tissue carboxy esterases (mainly CES1 ) and largely without cytochrome P450 -dependent degradation pathways. The resulting metabolite CNS-7054 has a 300-fold lower affinity for the receptor. and is therefore largely inactive.
Remimazolam is ultra-short-acting and has a terminal plasma half-life of 37 to 53 minutes, the mean half-life of the distribution (t 1 / 2α ) is between 0.5 and 2 minutes. In healthy volunteers, at least 80% and in colonoscopy patients 50% to 60% of the dose is excreted in the urine in the form of the pharmacologically inactive metabolite.
Side effects
Adverse effects such as reduced oxygen levels in the blood ( hypoxia ), reduced blood pressure ( hypotension ) and heart rate ( bradycardia ) require monitoring during sedation and during the recovery phase.
Studies
Approval was based on results from three randomized , multicenter clinical trials (Study 1 / NCT02290873, Study 2 / NCT02296892, and Study 3 / NCT02532647) in 969 adults who underwent brief interventions: Studies 1 and 3 were performed in participants, Undergoing a colonoscopy , Study 2 was performed in participants who underwent bronchoscopy . In the studies, the participants were randomly divided into three groups: one group received remimazolam, one group received placebo and one group received midazolam (a comparable, already approved drug with a sedative effect). The first two groups received double-blind treatment, each with the option of receiving additional midazolam if necessary. In the third group, all participants received midazolam only.
In all three studies, the participants also received a pain control agent.
Studies 1 and 2 compared participants who received Remimazolam with participants in the other two groups and measured the success of sedation based on the criteria given. Data from Study 3 were primarily used to assess the side effects of Remimazolam.
Others
In June 2020, the local ethics committee of the hospital in Milan received approval for the compassionate use of Remimazolam for sedation of patients with severe Covid-19 courses. The otherwise frequently used active ingredients midazolam and propofol had also become scarce in clinics in Italy.
Individual evidence
- ↑ Novel Drug Approvals for 2020 . FDA .
- ^ Remimazolam: First Approval In: Drugs. 2020, doi : 10.1007 / s40265-020-01299-8 . PMID 32274703 .
- ↑ a b c BYFAVO remimazolam besylate injection, powder, lyophilized, for solution . DailyMed .
- ↑ External identifiers or database links for remimazolambesilate. : CAS number: 1001415-66-2, EC number: 811-733-8 , ECHA InfoCard: 100.242.468 , PubChem : 23658607 , ChemSpider : 30790854 , Wikidata : Q27254247 .
- ↑ External identifiers from or database links to CNS-7054 : CAS number: 960305-91-3, PubChem : 46941174 , ChemSpider : 32697517 , Wikidata : Q27236793 .
- ↑ Drug Trials Snapshots: BYFAVO. US Food and Drug Administration, accessed July 26, 2020 .
- ↑ Instead of Propofol and Midazolam: Clinic is testing Remimazolam . In: Apotheke Adhoc . 2nd June 2020.