Ruby laser
The ruby laser was developed in 1960 by Theodore Maiman on the basis of the ruby grain as the first laser ever. He is one of the solid-state lasers ( solid state laser ). The most important emission line is at 694.3 nm (corresponds to a frequency of 432.1 THz).
construction
The active medium consists of ruby ( chrome aluminum oxide ), i.e. a single-crystalline Al 2 O 3 host crystal ( sapphire / corundum ), doped with chrome ions . Since the optimal degree of doping is around 0.03–0.05%, only specially manufactured rubies can be used (natural rubies have a higher chromium content). The rubies are made in stick form and the ends are polished very smooth. The roughness must be below half the laser wavelength, which is a major problem due to the extraordinary hardness of rubies.
In the past, the mirrors were formed directly on the crystal by vapor deposition of a layer of silver on the ends of the rod. Today the ends are usually given an anti-reflective coating and the mirrors are attached externally.
The crystal is optically pumped by means of xenon - flash lamps , since their light is absorbed particularly well. In very rare cases, continuous pumping also takes place, for example using mercury vapor lamps . In the first set-ups, a screw-shaped flash lamp was used with the ruby rod in the center. In modern lasers, however, the light from one or more rod-shaped lamps is usually focused directly onto the laser crystal by an elliptical mirror for more effective excitation.
functionality
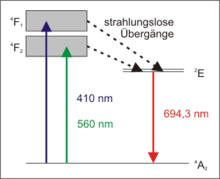
The ruby laser is a three-level laser. A large part of the electrons of the chromium ions is raised to one of the energy bands 4 F 1 or 4 F 2 by the optical pumping . A small part of it relaxes immediately back to the ground state 4 A 2 through spontaneous fluorescence emission .
The majority, however, merges into the metastable laser level 2 E in a radiationless transition . They stay there for a relatively long time. This can lead to population inversion in that more electrons are at the 2 E level than in the 4 A 2 ground state . From level 2 E, the electrons relax very slowly by themselves (in the range of about 3 milliseconds) with spontaneous emission of photons with a wavelength of 694.3 nm. These few photons can now, however, cause stimulated emission in the excited medium , thereby creating the coherent laser radiation arises and the excited electrons go into the ground state.
Due to the high ligand field splitting, the 2 T level above the 2 E level can be thermally occupied. Theoretically, this leads to a double line, which, however, is not observed. In contrast, the emission from both levels occurs in bis (N, N′ ‐ dimethyl ‐ N, N′ ‐ dipyridin ‐ 2 ‐ ylpyridin ‐ 2,6 ‐ diamino) chromium (III).
While most lasers can be operated in both pulse and cw ( continuous wave ) mode , the ruby laser can be found almost exclusively in the pulsed version, as this is where its efficiency is by far the highest. To operate it continuously, you have to keep the power very low, because the rubies can heat up very much when pumping and the heat is difficult to dissipate.
application
The ruby laser has largely lost its importance today, as its efficiency is comparatively low and the wavelength is accessible using other lasers.
In dermatology , due to the high pulse energy and good absorption of the laser wavelength by melanin, it is still used to treat pigment spots and to remove tattoos.
Literature and web links
- L. Bergmann, C. Schaefer Textbook of Experimental Physics Volume 3: Optik Walter de Gruyter Berlin, 10th edition 2004, ISBN 3-11-017081-7
- How a Laser Works (English)
Individual evidence
- ^ Lutz H. Gade: Coordination chemistry: GADE: KOORDINATIONS-CHEMIE O-BK . Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany 1998, ISBN 978-3-527-66392-7 , doi : 10.1002 / 9783527663927 ( wiley.com [accessed June 30, 2019]).
- ↑ Sven Otto, Markus Grabolle, Christoph Förster, Christoph Kreitner, Ute Resch-Genger: [Cr (ddpd) 2] 3+: a molecular, water-soluble, highly NIR-luminescent ruby analogue . In: Angewandte Chemie . tape 127 , no. 39 , September 21, 2015, p. 11735–11739 , doi : 10.1002 / anie.201504894 ( wiley.com [accessed June 30, 2019]).


