Rufinamide
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
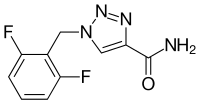
|
||||||||||||||||
| General | ||||||||||||||||
| Non-proprietary name | Rufinamide | |||||||||||||||
| other names |
1- (2,6-difluorophenyl) methyl-1 H -1,2,3-triazole-4-carboxamide |
|||||||||||||||
| Molecular formula | C 10 H 8 F 2 N 4 O | |||||||||||||||
| Brief description |
colorless powder |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| Drug information | ||||||||||||||||
| ATC code | ||||||||||||||||
| Drug class | ||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 238.19 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
240 ° C |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Rufinamide (trade name Inovelon ® ) is an anticonvulsant drug that is used in the treatment of Lennox-Gastaut syndrome , a particularly difficult form of epilepsy . It is only approved for additional therapy from the age of four.
pharmacology
Mechanism of action
Although the exact mechanism of action has not yet been explained, there is evidence that rufinamide exerts its anti-spasmodic effect by inhibiting voltage-dependent sodium channels on nerve cells. However, there is no evidence that it interferes with the GABA or the glutamate system.
Pharmacokinetics
After oral intake, the concentration in the blood reaches its maximum after about six hours. The level of the concentration achieved is not proportional to the amount administered, so that a dose-limited absorption behavior is assumed. The bioavailability depends on food intake and increases by about a third when administered with food. About a third of the active ingredient in the blood is bound to plasma proteins. Elimination takes place exclusively via conversion in the form of hydrolysis of the carboxy group with subsequent excretion via the kidneys. The half-life in blood plasma is 6–10 hours.
Side effects
The most common side effects reported in the clinical trial were headache, dizziness, tiredness, and drowsiness. Patients with Lennox-Gastaut syndrome in particular also reported very often nausea and vomiting. Other common side effects are upper and lower respiratory infections, loss of appetite with eating disorders and weight loss, anxiety, insomnia, double vision, blurred vision, nosebleeds, abdominal pain, constipation, diarrhea, rash, acne, back pain, gait disorders and oligomenorrhea. In the clinical development phase, the drug was discontinued in one fifth of cases because status epilepticus occurred.
Interactions
Other anti-epileptic drugs
Because the main area of application of rufinamide is Lennox-Gastaut syndrome, in which several anti-epileptic drugs are usually used, it is particularly important to consider the interactions with other active substances in this group of active substances. Rufinamide itself influences phenytoin by reducing excretion and increasing the concentration in the blood accordingly, so that a dose reduction for phenytoin may be necessary if used at the same time. The drug concentrations of carbamazepine , lamotrigine , phenobarbital , valproic acid or topiramate are unlikely to be influenced to a clinically relevant extent. However, simultaneous administration of valproic acid causes a significant increase in the rufinamide concentration, so that a dose reduction may be necessary. Carbamazepine, phenobarbital, phenytoin and vigabatrin , however, reduce the concentration of rufinamide.
Other active ingredients
Since the simultaneous administration of rufinamide with a contraceptive hormone preparation reduced the available amount of the estrogen component by 22% and of the progestin component by 14%, an additional method of contraception is recommended for women of childbearing potential who use oral contraceptives. This interaction is possibly due to the fact that rufinamide increases the activity of a certain subtype of the cytochrome P450 enzyme system, the CYP3A4. Therefore, other drugs that are broken down by this enzyme may also have a reduced effect.
Individual evidence
- ↑ a b Datasheet Rufinamide from Sigma-Aldrich , accessed on April 22, 2011 ( PDF ).
- ↑ Salunke, N .; Thipparaboina, R .; Chavan, RB; Lodagekar, A .; Mittapalli, S .; Nangia, A .; Shastri, NR: Rufinamide: Crystal structure elucidation and solid state characterization in J. Pharm. Biomed. Anal. 149 (2018) 185-192, doi : 10.1016 / j.jpba.2017.11.003 .
- ^ MA Rogawski: Diverse Mechanisms of Antiepileptic Drugs in the Development Pipeline . In: Epilepsy Res. 2006; 69 (3): pp. 273-294; PMID 16621450 ; PMC 1562526 (free full text).