Oxygen corrosion

As oxygen corrosion is called a corrosion process in which a metal is oxidized in the presence of water (humidity) by oxygen. In this redox reaction , oxygen is the oxidizing agent , as is the case with combustion in an oxygen atmosphere. However, the process takes place with the participation of water or an electrolyte solution without heating or flames.
Examples
Examples of such corrosion processes are the formation of rust on ferrous materials and the formation of patina on copper. However, because carbon dioxide and water or carbonic acid are also involved, the formation of copper patina can be assigned to both acid corrosion and oxygen corrosion .
requirements
The chemical reaction of the metallic material takes place under the influence or consumption of oxygen, i. H. the oxygen acts as an oxidizing agent. This process takes place primarily in alkaline and neutral solutions.
The prerequisites for oxygen corrosion are:
- neutral or alkaline electrolyte solution with dissolved oxygen (e.g. water in air )
- the metal redox couple must have a lower standard potential than the redox couple (E = 0.4 V).
procedure
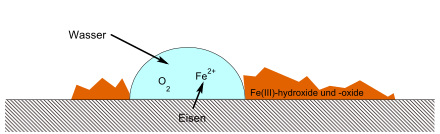
The oxygen molecules dissolved in the electrolyte solution react with water to form hydroxide ions, which can form oxides and hydroxides with the metal . This process is explained using the example of rust formation in the article rust .
In general, like any redox reaction, the course of oxygen corrosion can be divided into the sub-steps of oxidation (electron release) and reduction (electron acceptance).
- The metal is oxidized by releasing x electrons to the metal x cation, where x corresponds to the chemical valency ( ionic charge , oxidation number ) of the metal.
The overall reaction for a monovalent metal would be:
A short-circuited galvanic element can form during the corrosion process . This consists of two metals or metal pieces ( local elements ) with different electrode potentials ( cathode and anode ) and an electrolyte solution .
literature
- Gustav Peter, René Muntwyler, Marc Ladner: Building materials theory. (= Construction and Energy. Volume 3). 2., through and updated edition. vdf Hochschulverlag, 2005, ISBN 3-7281-3005-2 .
- Karl-Helmut Tostmann: Corrosion. Causes and Avoidance. Wiley-VCH Verlag, Weinheim 2001, ISBN 3-527-30203-4 .
Individual evidence
- ↑ The great table work. 2002, ISBN 3-464-57146-7 , p. 135.
Web links
- Corrosion damage caused by oxygen in the heating water - oxygen corrosion - (accessed on September 7, 2018)
- Rust and rust protection (accessed September 7, 2018)
- Basics of construction chemistry, electrochemistry, chemistry of metals (accessed September 7, 2018)
- Corrosion on metallic materials (accessed September 7, 2018)
- Corrosion and Corrosion Protection (accessed September 7, 2018)




