Galvanic cell

A galvanic cell [ galˈvaːnɪʃə t͡sɛlə ], galvanic element or galvanic chain is a device for the spontaneous conversion of chemical into electrical energy . Any combination of two different electrodes and an electrolyte is called a galvanic element, and they serve as sources of direct voltage . The characteristic value is the applied voltage . The capacity of a galvanic element is the product of the discharge current multiplied by time.
The name goes back to the Italian doctor Luigi Galvani . He discovered that a frog's leg nerve touched with instruments made of various types of metal triggers muscle twitching, since the redox system thus formed, as a galvanic element, builds up voltage so that current flows.
history
The evidence of the earliest galvanic cells in antiquity is controversial; it was possible to generate an electrical voltage with a replica of the approximately 1800 year old Baghdad battery . Whether the original antique clay pot was originally used for this purpose is not known and is a hypothesis.
The development of galvanic cells, as they are common today, began in the more recent modern times . The research work was triggered by observations made by Luigi Galvani around 1780: When he touched frogs' legs with two wires made of different metals, the muscles twitched. In 1790, Alessandro Volta showed that animal components are not necessary for the construction of battery cells. In the following years he used paper soaked in saline solution, which was stacked between metal plates made of copper and zinc and was known as the voltaic column .
The Leclanché element was patented by Georges Leclanché in 1866 and is one of the “wet batteries” that are no longer used today. It is an electrical battery (primary element), so it is not rechargeable and in its original form was equipped with liquid electrolyte. Technical improvements to the Leclanché element led to a gelled electrolyte and it is a forerunner of dry batteries such as the zinc-carbon cell and the alkaline-manganese battery that are common today.
The nickel-iron accumulator , one of the first rechargeable accumulators (secondary cell), was developed almost simultaneously and independently of each other by the American Thomas Alva Edison and the Swede Waldemar Jungner around 1900. It is considered the forerunner of the later developed and improved nickel-cadmium accumulator .
General
Galvanic cells are systematically divided into three groups:
- Primary cells , also known colloquially as a battery . It is characteristic that after joining the cell is charged and can be discharged once. The discharge is irreversible - the primary cell can no longer be electrically charged.
- Secondary cells , commonly known as a battery or a short battery called. After a discharge, secondary cells can be recharged by using a current that is opposite to the discharge. The chemical processes in the cell are reversible, limited by the number of cycles. The energy density of secondary cells is lower compared to primary cells at the same temperature.
- Fuel cells , also known as tertiary cells. With these galvanic cells, the chemical energy carrier is not stored in the cell, but made continuously available from the outside. This type of supply enables continuous and, in principle, unlimited operation.
The function of the galvanic cells is based on a redox reaction . Reduction and oxidation take place separately in a half-cell ( half- element). The circuit is closed by connecting the two half-cells with an electron conductor and an ion conductor . The voltage of the electric current can be calculated using the Nernst equation . It depends on the type of metal ( electrochemical series ), the concentration in the solution of the respective half-cell and the temperature. In contrast to electrolysis , for example in electroplating , electrical energy can be obtained in the galvanic cell, while electrolysis requires electrical energy.
When discharging galvanic cells, the negative pole is always the anode (= pole where the oxidation takes place), the positive pole is always the cathode (= pole where the reduction takes place). A donkey bridge for this is "OMA (oxidation, negative pole and anode)". During the charging process of the secondary cells, the chemical reactions at the poles are reversed: The oxidation takes place at the positive pole, which is why it then functions differently as an anode - accordingly the negative pole is the place of reduction and thus the cathode. The donkey bridge for the reaction during the charging process is accordingly: "OPA (oxidation, positive pole and anode)".
A galvanic cell supplies voltage until chemical equilibrium is reached. In contrast, a galvanic cell in which no current flows but an electrode voltage is present is in electrochemical equilibrium .
Oxidation takes place at the anode and reduction takes place at the cathode.
Examples
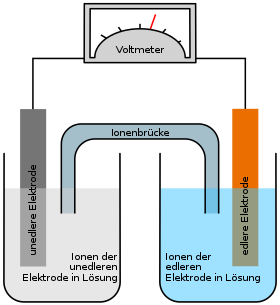
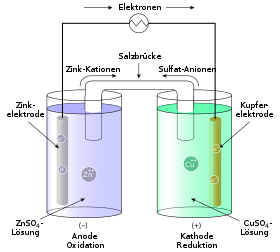
Whenever two different metals are in an electrolyte solution, a voltage (galvanic cell) is created. This is due to the tendency of the metals to go into solution and to form ions, the so-called solution tension . In addition to the Daniell element (copper / zinc), a galvanic element can also be created from copper and silver electrodes: The copper electrode is immersed in a copper sulfate solution, the silver electrode in silver nitrate solution, and these are connected by a wire (electron conductor) Voltmeter and an ion conductor .
If both electrodes are connected to each other via an electrical conductor, the different dissolution tension of the electrodes ensures that the reaction can continue. Since the redox potential of copper ( reducing agent ) is lower than that of silver ( oxidizing agent ), more ions go into solution at the copper electrode than at the silver electrode. This is why the negative charge in the copper electrode is higher than that in the silver electrode, so a voltage is created at which the electrons are “pushed” towards the silver electrode. This leads to the fact that the solution of the silver atoms is stopped, instead the excess electrons react with the Ag + ions of the silver nitrate solution and ensure that these stick to the silver electrode as normal silver atoms.
So silver ions are reduced to elemental silver at the silver electrode:
The silver electrode is therefore the cathode (electrode on which the reduction takes place) and the positive pole of the galvanic cell (since there is a lack of electrons here).
On the other hand, the following oxidation takes place on the copper electrode:
The copper electrode is the anode (electrode on which the oxidation takes place) and the negative pole of the galvanic cell (since there is an excess of electrons here).
A redox reaction takes place in the galvanic cell, the reaction parts of which, however, are spatially separated from one another.
If the two electrodes are now connected in an electrically conductive manner, a voltage is generated , but no current flows yet. The reason for this is that there is an excess of Cu 2+ ions in the copper sulfate solution and the solution has a strong positive charge, which prevents further copper atoms from being dissolved.
Something similar happens with the silver nitrate solution, which is negatively charged, since only the negatively charged nitrate ions remain from the neutral silver nitrate (while the positive silver ions attach to the silver electrode by taking up one electron each).
Silver nitrate solution: c [NO 3 - ] >> c [Ag + ]
Copper sulfate solution: c [SO 4 2− ] << c [Cu 2+ ]
That is why the electrode spaces are connected to one another via an ion bridge (salt bridge) , which is necessary to close the circuit. The ion bridge is often a U-tube that is filled with an electrolyte and the ends of which are provided with a membrane or a diaphragm . The ion exchange takes place via the salt bridge in order to counteract the charging of the individual cells. Another possibility of separating the electrode spaces from one another is a selectively permeable (selected permeable) membrane, which also enables charge equalization. The salt ions (in this case nitrate ions) migrate via the ion bridge from the cathode to the anode, i.e. from the silver half-cell to the copper half-cell.
There are also galvanic cells with two identical half-cells, which differ in their concentration, these are called concentration elements .
The deflagrator is also a galvanic cell.
Abbreviated notation
A galvanic cell is sometimes described in abbreviated form. The Daniell element shown graphically above would look like this in this notation:
Both half cells are separated in a galvanic cell by a diaphragm , i.e. a thin, semi-permeable membrane ( semipermeable membrane ). Almost exclusively the negatively charged anions are allowed to pass through this membrane . In the case of the Daniell element, these are SO 4 2− ions (sulfate ions), which are present in the salt solutions of both half-cells.
This diaphragm is represented in the abbreviated form by the double vertical line (||). To the right and left of this double line, the two half-cells of the galvanic cell and the reactions taking place in them are shown in abbreviated form. In addition, the concentration of the metal salt solution, i.e. the concentration of the dissolved metals in both half-cells, is given. The anode half-cell is usually on the left.
Web links
- Understandable, simple explanation of the galvanic cell with clear animation
- Galvanic element information
- Flash animation in English
- Video tutorial on the galvanic cell
- Video: What happens to the electrodes? - Current and voltage in electrolysis and galvanic cells . Jakob Günter Lauth (SciFox) 2013, made available by the Technical Information Library (TIB), doi : 10.5446 / 15232 .
Individual evidence
- ↑ Roland M. Horn: Mankind riddle : From Atlantis to Sirius , ISBN 978-3-7418-3767-8 ( partial preview online )
- ↑ Michael Buchholz: Subspace Identification for Modeling PEM Fuel Cell Stacks . KIT Scientific Publishing, 2010, ISBN 978-3-86644-477-5 , pp. 109 .
- ^ Elements Chemistry II - complete volume, ISBN 3-12-756700-6 , p. 157.