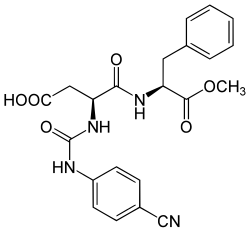
Super aspartame
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||
| General | |||||||||||||
| Surname | Super aspartame | ||||||||||||
| other names |
L -phenylalanine- N - {[(4-cyanophenyl) amino] carbonyl} - L -α-aspartyl-2-methyl ester |
||||||||||||
| Molecular formula | C 22 H 22 N 4 O 6 | ||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 438.43 g mol −1 | ||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Superaspartame is a synthetic sweetener that is chemically derived from aspartame . Compared to aspartame, the chemical structure has no free amino group because it is substituted by a ( p- cyanophenyl) carbamoyl radical. The substitution also creates a similarity to Suosan , another sweetener.
Superaspartame was discovered in 1982 by chemists at the University of Claude Bernard Lyon while searching for aspartame-based sweeteners.
The sweetness of superaspartame is around 14,000.
Another modification of the molecule by a sulfur group leads to thio-superaspartame .
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Christine Knopf: Relationship between structure and taste in aspartame and its analogs . diplom.de, 2000, ISBN 3-8324-2541-1 ( limited preview in Google book search).
- ↑ Hans-Dieter Belitz, Werner Grosch: Textbook of food chemistry . Springer-Verlag, 2013, ISBN 978-3-662-08304-8 ( limited preview in Google book search).
- ↑ Lyn O'Brien-Nabors: Alternative Sweeteners . Fourth ed. CRC Press, 2012, ISBN 978-1-4398-4615-5 ( limited preview in Google Book Search).
- ^ Hans-Dieter Belitz, Werner Grosch, Peter Schieberle: Textbook of food chemistry . 6th edition. Springer, Berlin 2008, ISBN 978-3-540-73202-0 , p. 454.
- ↑ Klaus Roth, Erich Lück: Calorie-free sweetness from the laboratory and nature . In: Chemistry in Our Time . tape 46 , no. 3 , June 2012, p. 168 , doi : 10.1002 / ciuz.201200587 .
- ↑ Klaus Roth: Chemical delicacies . John Wiley & Sons, 2014, ISBN 978-3-527-33739-2 ( limited preview in Google Book Search).