Cyanoacrylates
Under cyanoacrylate , cyanoacrylate or Alkylcyanacrylat is meant polymerizable , liquid at room temperature, chemical compounds ( monomers ), often referred to as adhesives are used. These are usually under the names Super Glue or Super Glue ( Engl. ) Or traded Germanized as "super glue".
properties
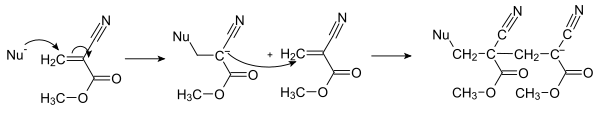
From a chemical point of view, these are esters of cyanoacrylic acid with alkyl chains of different lengths . Examples are methyl 2-cyanoacrylate , n-butyl cyanoacrylate and 2-octyl cyanoacrylate . The latter are now used in medical adhesives. In liquid form, cyanoacrylate consists of monomers such as methyl 2-cyanoacrylate (C 5 H 5 NO 2 ; molar mass: 111.1 g / mol) or ethyl 2-cyanoacrylate (C 6 H 7 NO 2 ; mol. Mass: 125 , 2 g / mol). The special property of this acrylic resin is its ability to undergo anionic polymerization , resulting in a poly (alkyl cyanoacrylate) . This is already started by low concentrations of hydroxide ions (reaction see fig.), So that the hydroxide concentration from autoprotolysis (10 −7 mol / L) of untreated water and the usual humidity are sufficient . Therefore, the monomers must be stored with the exclusion of air or with moisture-absorbing sodium silicate .
A cyanoacrylate polymer is a long chain that adheres very well to surfaces and is waterproof. The polymerization reaction is largely complete after a few minutes (depending on the moisture in the environment), and the fabric then reaches full strength after about 2 hours. Accelerators have been developed for special purposes that reduce the reaction to a few seconds at the expense of final strength. Organic solvents such as acetone or 2-butanone can loosen the adhesive without any problems. At low temperatures, the different coefficients of thermal expansion and the brittleness of the glass-like polymer, as with other adhesives, result in high mechanical stresses on the adhesive surface, and the frozen object “comes out of the glue”.
The polymerization reaction of cyanoacrylate adhesives is strongly exothermic . While sufficient heat dissipation is always guaranteed when gluing workpieces flat, self-ignition can occur if the adhesive is spilled on flammable materials. A few drops on a cotton shirt are not enough, but caution should always be exercised when handling cyanoacrylate.
History, application
Cyanoacrylate was first discovered by the American chemist Harry Coover , who worked at Eastman Kodak in New York on the development of optical prisms for weapons, during World War II in 1942. He experimented with the transparent acrylate plastics widely used in aircraft construction and replaced a methyl group with a cyano group. The extreme stickiness of the substance, which interfered with processing, initially prevented its industrial use, but this property was soon marketed profitably. Coover was working at the Tennessee Eastman company with Fred Joyner (1922–2011) and in 1951 he remembered his old discovery. Joyner first tried to use the material for jet cockpits as a heat-resistant clear coating and accidentally glued two lenses in an expensive laboratory device, which he ruined. Coover then recognized its importance as an instant adhesive. Cyanoacrylate was patented in 1956 and with Eastman 910 , the first adhesive on this basis came onto the market in 1958. In the TV show I've got a secret , Coover demonstrated the adhesive effect to the public by gluing two metal cylinders together, which the presenter could be lifted by shortly afterwards. Cyanoacrylates are now used as “superglues” in handicrafts and model making and are available in a wide range of viscosities and properties.
In 1964 , the Eastman company applied for medicine to the FDA , the US Food and Drug Safety Authority, to allow cyanoacrylate adhesives to bond human tissue and wounds. The special polymerization reaction (see above) helps in the event of accidents or seamless surgical interventions. Thanks to its ability to stop massive bleeding, cyanoacrylate quickly became an important tool for surgeons and has saved numerous lives to date. During the Vietnam War , cyanoacrylate sprays were used as a quick dressing for wounds , but due to possible skin irritation, these adhesives were not approved for civil use. It was only when the 2-octyl cyanoacrylate variant was developed in 1998 that the spray dressing was also able to spread in civil healthcare.
Cyanoacrylate-based adhesives have been approved in Europe as vein adhesives for forms of therapy on the venous system, for example for the treatment of varicose veins .
In forensic science, cyanoacrylate is used to make fingerprints visible. To do this, the liquid is heated and the vapors formed are reflected on fingerprints, which, however, must still have a certain amount of residual moisture. The fingerprint is then visible as a white pattern.
Individual evidence
- ↑ Spontaneous Combustion! - How To , Video at Metacafe.
- ↑ Super Glue . In: Inventor of the Week . Massachusetts Institute of Technology. September 2004. Accessed February 27, 2014.
- ↑ Lowe, Das Chemiebuch, Librero 2017, p. 328.
- ↑ Ulf Thorsten Zierau: Vein glue for varicose veins - upgrade factual check. In: saphenion.de. Practice Clinic for Vascular Diseases and Vein Center Berlin Rostock, November 26, 2017, accessed on November 29, 2017 .
